CONTENTS
- Rapid Reference 🚀
- Initial evaluation
- Non-intubated patients
- Intubation
- Intubated patients
- Other topics:
- Podcast
- Questions & discussion
- Pitfalls
non-intubated asthmatic
basic medication package:
- Bronchodilation: 📖
- Stacked albuterol nebs (2.5-5 mg q20) or continuous neb (10-15 mg/hr).
- Ipratropium (may use 1.5 mg over first hour, then 0.5 mg nebulized q4-6 hr).
- Methylprednisolone 125 mg IV x1 (or equivalent steroid). 📖
- Magnesium sulfate: 2 grams (8 mM) IV. 📖
BiPAP +/- sedation
- BiPAP is preferred, if possible. 📖
- Sedation, if needed: 📖
- Start dexmedetomidine infusion at maximal rate (down-titrate as takes effect). This may be helpful as an anxiolytic agent, even if the patient is able to tolerate the BiPAP mask.
- May use small doses of opioid while waiting for dexmedetomidine to take effect, if severely dyspneic (e.g., fentanyl 25 mcg IV PRN).
- (If wholly unable to tolerate BiPAP, may use high-flow nasal cannula or heliox 📖).
epinephrine infusion 📖
- Indications for epinephrine infusion:
- Failure of inhaled bronchodilators.
- Unable to tolerate inhaled bronchodilators (re: coughing).
- Bradycardia related to dexmedetomidine.
- Start 5 mcg/min, titrate 1-10 mcg/min (peripheral IV is fine).
- Alternative systemic bronchodilators:
- Terbutaline (0.25 mg SC, q15-30 min x3 doses PRN).
- Glycopyrrolate (0.2 mg IV; less evidence).
intubated asthmatic
basic medication package:
- Bronchodilators: Frequent albuterol nebs (2.5 mg q20 min) or continuous neb (10-15 mg/hr). 📖
- Steroid: Methylprednisolone ~2 mg/kg/day may be reasonable. 📖
- Magnesium sulfate: 2 grams (8 mM) IV. 📖
- Montelukast 10 mg/day enterally. 📖
- Bicarbonate if there is difficulty achieving an adequate pH. It is often helpful to push the bicarb up to a high-normal level (29 mM) or moderately elevated level (35 mM). 📖
- Analgosedation: 📖
- High-dose propofol infusion is very helpful (~60-80 ug/kg/min).
- Fentanyl boluses PRN (& infusion if needed for vent synchrony).
- Acetaminophen scheduled (usually 1 gram PO q6hr).
- Pain-dose ketamine (~0.25 mg/kg/hr).
ventilator settings 📖
- Key is a low respiratory rate (~12-14 breaths/min).
- Target tidal volume ~6-8 cc/kg.
- Permissive hypercapnia: target pH >~7.15 if able. 📖
enteral nutrition 📖
- Initiate early to reduce the risk of propofol infusion syndrome.
if refractory: dissociative ketamine gtt 📖
- Ketamine dose: Load with 1-2 mg/kg then infuse at 1-2 mg/kg/hr.
- Add glycopyrrolate to avoid bronchorrhea & dilate bronchi (e.g., 0.2 mg IV q6hr).
diagnoses that are often confused with asthma (& clues to help suggest them)
- Anaphylaxis:
- Involvement of other organ systems (e.g. urticaria, vomiting, shock).
- Stridor due to angioedema.
- Vocal cord dysfunction (or other upper airway obstruction):
- Wheezing audible from across the room (this is not a feature of severe asthma!).
- Pneumothorax:
- Thoracic ultrasound shows lack of lung slide on affected side.
- If lucky may see traditional signs (e.g., subcutaneous crepitus).
- Chest X-ray will reveal pneumothorax.
- Pneumonia:
- Historical features (sputum production, fever).
- Infiltrate on chest X-ray; focal B-lines seen on thoracic ultrasound.
- Heart failure (“cardiac asthma”):
- Key finding = Diffuse B-line pattern visible on thoracic ultrasonography.
- Other supportive features may include peripheral edema, echo c/w heart failure.
- Anxiety / Panic:
- ABG/VBG demonstrates respiratory alkalosis.
- Room air ABG demonstrates normal A-a gradient.
evaluation to determine diagnosis
- History (to the extent that it is available).
- Physical examination
- Asthma: Wheeze loudest over lung fields.
- Upper airway obstruction: Wheeze loudest over throat.
- Bedside ultrasonography
- Thoracic ultrasonography: Exclude pneumothorax or heart failure.
- Cardiac ultrasonography: Evaluate volume status, function.
- Chest X-ray
- Not immediately essential if bedside ultrasonography performed.
- Useful to evaluate for pneumonia and obtain baseline for comparison.
indicators of severe asthma include:
- Respiratory rate >30.
- Work of breathing, accessory muscle use (patient looks bad).
- Inability to speak in full sentences.
- Tripoding, inability to lie flat.
- Paradoxical breathing (abdomen and chest move synchronously).
- Wheezing may be absent if patient is moving very little air (“silent chest” is an ominous finding).
- Worsening hypoxemia (asthma alone shouldn't generally cause significant hypoxemia, so worsening hypoxemia often indicates mucus plugging or atelectasis due to progressive respiratory failure).
- Bradycardia is generally a harbinger of impending respiratory arrest.
- Somnolence is very worrisome (unless the patient received sedation).
Although patients will vary, the central pathophysiology often involves tachypnea leading to gas-trapping in the chest (autoPEEP) which exacerbates dyspnea in a vicious cycle:
high dose inhaled albuterol
- Patients should be started on high-dose albuterol. Two options are roughly equivalent:
- (a) Stacked nebs: 2.5-5 mg via nebulizer Q20 minutes back-to-back.
- (b) Continuous nebulized therapy (10-15 mg/hour initially).
- As patients improve, this should gradually be weaned down and spaced out.
inhaled ipratropium
- Addition of ipratropium to initial therapy (e.g. 1.5 mg inhaled over the first hour of therapy) may be helpful. Subsequently 0.5 mg may be nebulized Q4-6 hours.
epinephrine
- Not supported by any high-quality evidence.
- Indications may include:
- (a) Patient unable to tolerated inhaled bronchodilators (e.g. due to coughing).
- (b) Failure to improve with inhaled bronchodilators.
- Intramuscular epinephrine:
- Dose is 0.3 – 0.5 mg IM, may repeat 1-2 times Q20 minutes.
- Not preferred: lack of control over the dose; unable to down-titrate if complications occur.
- Intravenous epinephrine infusion:
- Start at 5 micrograms/minute, rapidly titrate to effect over roughly 1-15 micrograms/minute range. Down-titrate as soon as possible.
- Intravenous administration provides more flexibility than IM epinephrine (e.g. if hypertension or excessive tachycardia occurs, can down-titrate or stop it immediately).
- Epinephrine is safe for peripheral administration (it doesn't require a central line).
- (More on IM vs IV epinephrine here.)
other systemic beta-2 agonists
- Terbutaline 💊 is a reasonable option, which has gained some popularity during the COVID pandemic (as an alternative to nebulized albuterol, to avoid aerosolization).(32340824) The main limitation surrounding terbutaline is logistic (due to delays in obtaining the medication from the central pharmacy). If available, the dose of terbutaline is 0.25 mg, q15-30 minutes doses PRN, up to three doses.
IV anticholinergic agents?
- IV anticholinergic agents are a sensible consideration for the same reason that IV beta-agonists are: in severe patients with extreme obstruction or inability to tolerate nebulized medications the systemic route may be more effective.
- Glycopyrrolate 💊 0.2 mg IV has been reported to be effective in one case report.(3619169) Although the evidence level is obviously very low, mechanistically this makes sense. The utility of inhaled glycopyrrolate is better established than IV glycopyrrolate.(16236844, 3392363, 3792086, 2225951) This should be used only with close monitoring of hemodynamics (there may be a risk of tachycardia if used in combination with beta-agonists).
potential indications?
- Respiratory rate >25-30/min.
- Significant work of breathing.
initial settings
- Start with standard settings (10 cm inspiratory pressure / 5 cm expiratory pressure).
- Titrate FiO2 to keep the saturation ~93-95%. Asthmatics shouldn't require much oxygen (if the FiO2 requirement is high, look for an alternative or additional diagnosis).
- A prospective RCT suggests that excessive oxygen administration may impair ventilation-perfusion matching and thereby worsen hypercapnia.(21597111)
- The role of various settings is shown below.(7663804, 29131536, 30996631) Different patients may have different physiologies and thus individual titration to effect can be helpful.
 difficulty tolerating BiPAP
difficulty tolerating BiPAP
- Various sedation strategies are usually successful at overcoming this (below: 📖). It's generally worth making a real effort to get patients on BiPAP.
- If patients genuinely cannot tolerate BiPAP, other options are HFNC or heliox: 📖
evidentiary basis
- The largest published series by Bond et al. describes 186 patients treated with noninvasive ventilation, of whom only 8 required intubation. Most patients were treated with CPAP alone with ~10 cm pressure. Many patients with poor mental status were successfully treated with noninvasive ventilation, proving that altered mental status alone is not an absolute contraindication to noninvasive support.(29131536)
- BiPAP is supported by robust data in COPD. Since the physiology of asthma and COPD are similar (with some patients having asthma/COPD overlap), this evidence may be applicable to asthma as well.
 The approach to sedation depends on how sick the patient is and how much time you have to titrate medications to effect. A general concept of how this might work is provided above, but there is no good data on any of this. Sedation plays a dual role of facilitating the use of BiPAP and also eliminating anxiety-driven tachypnea (which may exacerbate gas trapping in the chest).
The approach to sedation depends on how sick the patient is and how much time you have to titrate medications to effect. A general concept of how this might work is provided above, but there is no good data on any of this. Sedation plays a dual role of facilitating the use of BiPAP and also eliminating anxiety-driven tachypnea (which may exacerbate gas trapping in the chest).
dexmedetomidine is usually the best agent
advantages of dexmedetomidine
- It's a titratable sedative, so it can be adjusted over time to match the patient's needs.
- It doesn't suppress respiratory drive (so if patient's look better on dexmedetomidine, this generally reflects true improvement).
- It may have bronchodilatory properties itself, which could directly improve asthma. (26716866, 14739811)
drawbacks
- (1) May not be widely available.
- (2) Dexmedetomidine can take a little while to work:
- Dexmedetomidine may be started at a high rate (e.g. 1.4 mcg/kg/hr) and then down-titrated over the next hour as levels accumulate.
- For patients with substantial anxiety, a small bolus of fentanyl or haloperidol may be used while dexmedetomidine is taking effect (more on these below).
More on dexmedetomidine here: 📖
IV haloperidol or IV droperidol
advantages:
- Lack of respiratory suppression.
- No disinhibitory or paradoxical effects.
- Anti-nausea properties.
- Reasonably fast onset of action.
- Widely & immediately available.
disadvantages:
- Short-acting, will require ongoing re-dosing.
role?
- May be useful for initial management.
- Not an ideal ongoing solution for sedation. Thus, patients may be eventually transitioned over to dexmedetomidine.
benzodiazepines
- Benzodiazepines can sometimes be helpful, particularly for patients who chronically use benzodiazepines (and are known to respond favorably to them).
- However, in many patients benzodiazepines will induce delirium or paradoxical agitation – which can be extremely problematic.
- Overall, the clinical effects of benzodiazepines are hard to predict, limiting their application.
opioids
potential advantages of opioids:
- They may help break a vicious cycle of hyperventilation-induced gas trapping.
- Advantage compared to dexmedetomidine = immediate action.
- Advantage compared to ketamine = no risk of post-recovery emesis.
- A rapid-acting, titratable opioid is usually most useful (e.g., IV fentanyl). Morphine should be avoided because histamine release could theoretically worsen bronchospasm.
potential roles for opioids:
- For a patient with moderate severity disease, as a temporary bridge while waiting for dexmedetomidine to take effect.
- If patients have pain for some other reason, then use of a low-dose opioid could be beneficial for pain (with mild blunting of the respiratory drive as a bonus effect).
- (Evidentiary basis: There isn't any high-level evidence supporting opioid. However, studies have described the use of opioid to facilitate sedation on noninvasive ventilation.)(25699177, 26164393)
opioid changes the way patients must be monitored:
- Without opioid on board, respiratory rate may be used as a general index of how sick patients are (as a reflection of ventilatory efficiency). Opioid directly suppresses respiratory rate, making this metric potentially unreliable.
- Approaches to monitoring in a patient on opioids may include:
- (1) Monitoring of mental status (worsening mental status over time suggests hypercapnia, but this is a late finding)
- (2) Monitoring of tidal volume and minute ventilation on BiPAP (presuming an adequate mask seal).
- (3) Periodic ABG/VBG monitoring may be needed. Please note that the goal of this monitoring isn't to see an immediate improvement in CO2, but rather to make sure that the patient isn't becoming progressively more hypercapnic.
delayed sequence intubation (DSI) with dissociative ketamine
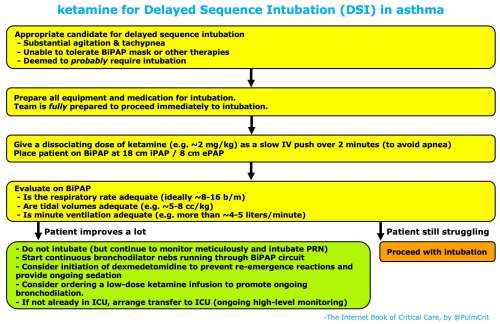
- This should only be done rarely, on extremely ill patients who look like they will likely require intubation. 🌊
- Some patients will improve immediately with ketamine, avoiding intubation. This is probably due primarily to ketamine's breaking a cycle of agitation-induced tachypnea (although ketamine-induced bronchodilation and facilitation of BiPAP probably help too).
- If patients don't improve rapidly following ketamine administration, this will result in excellent pre-oxygenation prior to intubation (so it's still a useful strategy).
- Ketamine may cause emesis, but this occurs as patients are waking up from dissociation. Close monitoring is required during this period.
For patients who are unable to tolerate BiPAP, other support options include heliox or HFNC (high-flow nasal cannula).
heliox
- Limitations:
- May not be available.
- Heliox is formulated as a 70:30 or 80:20 mixture, so this isn't an option for patients requiring >30% FiO2.
- If heliox is an option, it might be preferable to HFNC (with somewhat better evidentiary support).(35218742)
high-flow nasal cannula
- Useful in patients for whom BiPAP or heliox aren't options.
- Washout of dead space may reduce the work of breathing. 📖
- Patients benefit mostly from the increased flow rate (not the oxygen delivery).
- (#1) Loading dose: methylprednisolone 125 mg IV.
- (#2) Initial maintenance dose:
- No solid evidence on this.
- ~125 mg IV methylprednisolone daily seems reasonable (or roughly 2 mg/kg daily).
- One RCT found that 100 mg methylprednisolone was equally effective to 500 mg when administered in the emergency department.(7781346) This suggests that optimal efficacy may be reached by a dose of ~100 methylprednisolone, and much higher doses may not be beneficial.
- (#3) Taper:
- Once patients are making a solid recovery, steroid dose can be decreased 60 mg/day prednisone.
- If this is tolerated, a reasonable taper might be to decrease to 40 mg/day prednisone for 5 days, then stop.(35218742)
IV magnesium
- This is a source of ongoing controversy. IV magnesium is safe and possibly effective, but hasn't been borne out in large adult RCTs such as the 3MG trial.(25759905, 30633478, 24731521) A recent article summarizing available evidence is here. Some additional discussion of this may be found here.
- This therapy is reasonable. However, it's probably not enormously effective and definitely should not interfere with other treatments.
- Administration of two grams of magnesium over 20 minutes is reasonable (this dose of magnesium is extremely safe).
leukotriene inhibitors (montelukast)
- Used in chronic asthma due to bronchodilatory and anti-inflammatory properties.(20085924, 15539716)
- Some very weak evidence suggests a possible benefit from oral montelukast (10 mg daily).(20956393)
- The use of oral montelukast is limited in acute status asthmaticus because patients should be NPO.
- If intubation is required, enteral montelukast might be a reasonable consideration, especially if:
- i) Patient is on chronic montelukast.
- ii) Asthma is related to NSAIDs or aspirin (this type of asthma involves leukotriene overproduction).
Progress is evaluated by trending several parameters:
(#1/3) work of breathing & respiratory rate
- Ideally respiratory rate will decrease to about 10-20 breaths/minute.
- Persistent tachypnea (e.g. respiratory rate >>25 b/m consistently) is worrisome with regard to risk of worsening autoPEEP and respiratory fatigue.
- If the respiratory rate remains elevated, consider adjusting the BiPAP pressures or adding sedation (dexmedetomidine).
(#2/3) monitoring on BiPAP: tidal volume & minute ventilation
- These seem to be reasonably accurate, as long as there isn't a large mask leak.
- Tidal volumes should ideally be reasonably large (e.g. >5 cc/kg). If the tidal volumes are small (e.g. ~2-3 cc/kg) then the patient may get almost no effective ventilation (discussed further below 📖).
- Minute ventilation should be reasonably large (e.g. at least ~4-5 liters/minute, ideally higher).
(#3/3) either normal mental status *or* absence of worsening hypercapnia on ABG/VBG
- ABG/VBGs are not needed for patients with normal mental status who otherwise seem to be doing well.
- For patients with abnormal mental status due to sedation, periodic blood gas (VBG or ABG) may provide reassurance that patient is ventilating adequately.
- Note that the goal of initial treatment is really to reduce the work of breathing, not immediate improvement in the ABG/VBG.
- If CO2 is increasing substantially (e.g. >20 mm increase), this is worrisome with regard to treatment failure.
- Treatment pseudo-failure is when the patient's lungs improve, but lactic acidosis occurs due to beta-agonist stimulation from albuterol and/or epinephrine. If severe, acidosis may trigger a compensatory respiratory alkalosis which will worsen dyspnea.
- Diagnosis of pseudo-failure:
- Lungs sound better.
- Patient improves initially, then deteriorates.
- Labs show metabolic (rather than respiratory) acidosis.
- If measured, lactate is elevated.
- Management: these patients generally do well if you just cut back on the beta-agonists (e.g. stop epinephrine, wean albuterol as tolerated).
There is no high-quality evidence regarding when intubation is indicated. This is a clinical decision, made primarily based on serial evaluation by experienced practitioners.
indications for intubation might include the following:
- Progressive deterioration despite maximal noninvasive therapies, e.g.:
- Worsening work of breathing and impending respiratory exhaustion.
- Significantly worsening hypercapnia.
- Impending cardiopulmonary arrest (suggested by bradycardia, severe respiratory acidosis, very poor air movement, obtundation).
- Mental status alteration with inability to protect airway (noting, however, that published case series have reported high success using noninvasive ventilation, even in patients with altered mental status).(29131536)
risk of hemodynamic deterioration
- High airway pressures often cause hypotension after intubation, so prepare for this.
- Consider administration of volume if there is any evidence or history of hypovolemia.
- Start an epinephrine infusion prior to intubation if hemodynamics are tenuous (this may be beneficial for bronchospasm as well 📖).
use a large ETT
- Multiple reasons:
- Will reduce airflow resistance, facilitating ventilation.
- May facilitate procedures if necessary (e.g. bronchoscopy).
- Most sources recommend using at least a #8 ETT in adults (but this may vary depending on the patient's height).(29105540)
avoid aggressive bag-mask ventilation!
- Aggressive bagging will rapidly precipitate severe gas-trapping in the lungs.
- Gas-trapping may cause pneumothorax or hypotension.
asthmatics should be difficult to ventilate
- If the patient is easy to ventilate (e.g. with a normal peak inspiratory pressure), then they don't have status asthmaticus. There must be some other form of upper airway obstruction, which has been alleviated by placement of the endotracheal tube.
- The most common cause is vocal cord dysfunction.
- Other possibilities include epiglottitis or neck abscess.
- If the diagnosis isn't clear (e.g. if the patient doesn't have vocal cord dysfunction), then consider a CT scan of the neck to exclude anatomic problems.
permissive hypercapnia
When hypercapnia is imposed by rapidly deteriorating lung mechanics, as in acute severe asthma, there is little choice but to accept a brutal, at times extreme rise in pCO2 in order to prevent life-threatening barotrauma… In patients with sudden asphyxic asthma, our experience is that pCO2 much in excess of 80 mm (up to 200 mm) must often be accepted. -Feihl F and Perret C AJRCCM 1994 (7952641)
basic concepts of permissive hypercapnia
- Permissive Hypercapnia is the core of safe ventilation in asthma. This simply refers to the concept that it's often safer to allow the pH to remain low, rather than trying to strictly control the pH (since normalizing the pH would typically lead to an unacceptably high risk of pneumothorax, or autoPEEP causing cardiovascular collapse).
- Hypercapnia is generally well tolerated, especially in asthmatics (who tend to be young, with good hemodynamic reserves).
- The purpose of intubation in asthma is not to reduce the CO2 – it's really just to keep the patient alive long enough for their lungs to improve.
- Intubation keeps patients from dying due to respiratory exhaustion (which eventually leads to apnea and hypoxemia).
- The pCO2 is often higher on the ventilator than it was before the patient was intubated!
contraindications to permissive hypercapnia:
- Active CNS disease with elevated intracranial pressure.
- Significant pulmonary hypertension with cardiac compromise (hypoperfusion or hypotension).
- Pregnancy (perhaps).
the optimal pH target is unknown
- There is no specific pH below which patients will deteriorate – this may vary between patients depending on their physiology.
- Young patients can often tolerate respiratory acidosis with pH <7 surprisingly well.
- Different providers have different pH targets, which are arbitrary and not evidence-based. It's nice to see the pH >7.15 if possible, but ultimately this is a judgement regarding balancing the risks-vs-benefits of more aggressive ventilation vs higher pH.
autoPEEP is the main problem with ventilation
basics
- autoPEEP refers to trapping gas within the lungs during respiration. This occurs if one breath can't be fully exhaled prior to the next inhalation. The net result of this trapped gas is to create additional positive pressure (“auto PEEP”) within the chest, which is higher than the PEEP provided by the ventilator (the “set PEEP”).
- autoPEEP is inevitable with severe asthma. The goal is to minimize it as possible.
evaluation of autoPEEP
- If the expiratory flow curve never comes close to zero, then autoPEEP is present.
- Some degree of autoPEEP will be seen in most intubated asthmatics.
- An end-expiratory breath hold maneuver can measure the autoPEEP directly (although this is only accurate among patients who are passive on the ventilator).
- AutoPEEP will make it more difficult for the patient to trigger the ventilator (in order to trigger a breath, they need to overcome the autoPEEP). Thus, difficulty triggering the ventilator may be an indirect sign of autoPEEP.
- Hypotension may be a sign of severe autoPEEP.
management of autoPEEP
- (1) 🏆 Reduction in the respiratory rate is the most effective strategy.
- Respiratory rate should be <20 breaths, and generally closer to ~10-14 b/m.
- (2) Decrease the inspiratory time (e.g. using a higher flow rate if you're using volume-cycled ventilation).
- (3) Decreasing the tidal volume: Tidal volumes should ideally be maintained around 6-8 cc/kg. Dropping the tidal volume much below 6 cc/kg will cause problems with dead space ventilation:
- Dead space is volume which enters the lungs but doesn't participate in gas exchange. The amount of dead space is the sum of the anatomic dead space (gas going into and out of the trachea and large bronchi) plus the physiologic dead space (gas going into and out of non-functional alveoli).
- The anatomic dead space is roughly fixed, at ~2.2 ml/kg. So right off the bat, ~2 cc/kg of each breath is wasted in ventilating the anatomic dead space (this achieves nothing for the patient).
- Asthmatic patients may have increased physiologic dead space, causing their total dead space to be relatively high (e.g. ~3 cc/kg). This means that if the tidal volumes are very low (e.g. 3-4 cc/kg), then the vast majority of ventilation will be wasted! This may cause the patient to be profoundly hypercarbic – even though the minute ventilation isn't terribly low.
how should the extrinsic PEEP be set?
This is a topic of considerable debate, although it probably doesn't make a big difference. If you don't want to deal with this and just set the PEEP at 5-8 cm that's probably fine.
clarifying some terminology:
- Set PEEP is the amount of PEEP dialed into the ventilator.
- Intrinsic PEEP is the actual intrathoracic pressure at end-expiration (the “true” PEEP).
- Intrinsic PEEP may be measured by performing an end-expiratory breath hold maneuver. Unfortunately, this is only accurate in a patient who is passive on the ventilator.
benefits of set PEEP:
- May help stent open the airways during exhalation (otherwise the airways may tend to be compressed by adjacent lung tissue).
- In order to trigger a breath from the ventilator, the patient needs to suck the pressure in their lungs down from intrinsic PEEP to below the set PEEP. Thus, the work of triggering the ventilator is proportional to the difference between the Intrinsic PEEP and the Set PEEP. Increasing the Set PEEP a bit will make it much easier for the patient to trigger the ventilator.
how to set the PEEP?
- If the patient is not passive on ventilator (e.g. patient is triggering the ventilator), just use 5 cm PEEP.(26033128)
- If the patient is passive on the ventilator, it may be reasonable to carefully titrate the PEEP. This begins with a measurement of the intrinsic PEEP. It may be reasonable to increase the PEEP to a level equal to ~75% of the intrinsic PEEP. Mechanics should be monitored during titration for signs of excess PEEP, which would be:
- Volume-cycled ventilation: Increasing PEEP causes an increase in plateau pressure.
- Pressure-cycled ventilation: Increasing PEEP causes a decrease in tidal volume.
pressure-cycled vs. volume-cycled ventilation
- Either mode may be used; this is largely a matter of familiarity.
- Pressure-cycled ventilation has the advantage of achieving better control over alveolar pressure, but this comes at the expense of losing control over tidal volume. Alternatively, volume-cycled ventilation has the advantage of controlling tidal volume, but this comes at the expense of losing control over alveolar pressure.
volume-cycled ventilation
initial settings
- Tidal volume: 8 cc/kg.
- Respiratory rate: 12 breaths/minute.
- Flow rate: 80 liters/minute (set this high in asthma to reduce inspiratory time).
- FiO2: start ~60%, titrate for an oxygen saturation >88%.
- PEEP: start at 5 cm.
- Peak pressure alarm may need to be increased to prevent its constantly going off.
monitoring for safety
- (1) Plateau pressure should be monitored if possible (if the patient is passive on the ventilator). This may be achieved by an end-inspiratory breath hold maneuver. Plateau pressure should ideally be under ~35 cm.
- (2) Follow oxygenation; rapidly deteriorating oxygenation suggests an acute problem such as pneumothorax.
pressure-cycled ventilation
initial settings
- Inspiratory pressure: ~35 cm (titrate against tidal volume to achieve ~6-8 cc/kg)
- Respiratory rate: 12 breaths/minute
- Inspiratory time: 1 second
- FiO2: start ~60%, titrate for an oxygen saturation >88%
- PEEP: start at 5 cm
monitoring for safety
- (1) Tidal volume should be monitored. Ideally this should be ~6 cc/kg (but it may be lower, ~4-5 cc/kg, initially).
- Falling tidal volume may be a sign of pulmonary deterioration (e.g. mucus plugging or pneumothorax).
- Increasing tidal volume may be a sign of clinical improvement.
- If the tidal volume is unnecessarily high, consider decreasing the inspiratory pressure.
- (2) Follow minute ventilation.
- Initially this will be low (e.g. ~4-6 liters/minute).
- With improvement, the minute ventilation may increase to a normal range (e.g. ~7-8 liters/minute).
- (3) Follow oxygenation; rapidly deteriorating oxygenation suggests an acute problem, such as pneumothorax.
“we're bagging the patient because the ventilator isn't working”
Sometimes an outside hospital may call regarding an asthmatic who has been intubated and is unable to ventilate. The problem is that airway pressures required to ventilate a patient in status asthmaticus will trigger alarms on standard ventilator settings. The following is an approach to set the ventilator over the phone, in such a way which is safe for the patient. Whenever possible, asthmatic patients should not be transferred with ongoing bag-mask ventilation (this will almost invariably lead to gas-trapping and potentially pneumothorax).
(#1) Sedate the patient deeply (ideally with IV ketamine boluses +/- infusion).
(#2) Paralyze the patient with a long-acting agent (e.g. vecuronium or rocuronium).
(#3) Set the patient on a pressure-cycled ventilation (PC) with the following settings:
- Respiratory rate of 12 breaths/minute.
- Inspiratory time (I-time) of 1 second.
- Inspiratory pressure of 40 cm, with a PEEP of 5 cm.
- FiO2 initially at ~60%, titrate against the patient's oxygen saturation.
These settings will ensure that the patient doesn't receive excessive pressures, but they won't ensure that the patient receives a sufficient tidal volume. This is prioritizing lung protection over CO2 normalization (more on this below). Attention is needed to ensure that adequate ventilation is obtained (e.g. tidal volumes of at least ~4 cc/kg and a minute ventilation of at least ~3-4 liters/minute). If this is achieved, then the patient can generally be stabilized sufficiently to transfer.
rationale for bicarbonate administration
- Bicarbonate administration in respiratory failure (e.g. ARDS or asthma) hasn't been investigated rigorously, so the benefit is unknown. Bicarbonate administration makes sense for the following reasons:
- (1) Acceleration of normal compensatory mechanisms: Normally, the kidneys will retain bicarbonate in response to a respiratory acidosis. This renal compensation helps stabilize the pH – but it takes days. Exogenous alkali administration merely speeds up this normal physiological process.
- (2) Reduced respiratory drive: Improving the pH should decrease the respiratory drive, which may reduce the amount of medication required to keep the patient comfortable on the ventilator.
- (3) Avoidance of ventilator & ECMO insanity: Low pH will induce some providers to do all sorts of bizarre interventions with the ventilator (e.g. prolonged paralysis) and refer patients for ECMO. Making the pH numbers look better will help the patient avoid iatrogenic harm.
indications
- Bicarbonate may be considered whenever there is difficulty achieving an adequate pH and the patient's bicarbonate level is below the level consistent with chronic compensation (table above).
- ⚠️ Note, however, that if the patient has a high-anion gap metabolic acidosis due to lactic acidosis or ketoacidosis, then the underlying cause of these disorders needs to be treated. For example, asthmatics may develop lactic acidosis due to excessive beta-agonist use – this shouldn't be treated with bicarbonate.
- A normal serum bicarbonate level is 22-29 mM. Thus, pushing the serum bicarbonate up to a high-normal level (~29 mM) is quite reasonable for any patient.
- In practice, it's often helpful to increase the bicarbonate to ~35 mM.
technique
- Selection of hypertonic versus isotonic bicarbonate is discussed here: 📖
- If isotonic bicarbonate is used, this may be combined with gentle diuresis to prevent volume overload (but be careful to avoid hypokalemia).
Fairly deep sedation is needed initially. The best agents to use here are drugs which directly suppress the respiratory drive (opioids and propofol). A reasonable starting place is often a combination of propofol, opioid, and ketamine:
propofol
- Propofol is the cornerstone of initial sedation for the intubated asthmatic, with numerous benefits:
- Patients usually require moderate-to-high doses (e.g. ~60 mcg/kg/min).
- Consider early initiation of enteral nutrition to reduce the risk of propofol infusion syndrome.
opioid
- Morphine should be avoided, because it may increase histamine release and cause bronchospasm (fentanyl or hydromorphone is preferable).
- Opioid dosing will depend to a large extent on how the patient responds to propofol:
- Many patients can be rendered comfortable on a moderate dose of propofol with PRN doses of opioid.
- Some patients may require considerable doses of both propofol and opioid to suppress their respiration enough to synchronize with the ventilator.
- If a fentanyl infusion is used, the lowest possible dose should be utilized (ideally <100 mcg/hr). Note that 100 mcg/hr fentanyl is equivalent to 480 mg/day of oxycodone (a lot of opioid!).
pain-dose ketamine (0.1-0.3 mg/kg/hr)
- This is generally useful here for several reasons:
- (1) May reduce the incidence of tolerance and opioid-induced hyperalgesia among patients on opioid infusions (this may mitigate the harm caused by opioid infusions to a certain extent).
- (2) Provides pain relief.
- (3) May provide some bronchodilation.
- Propofol decreases the likelihood of partial dissociation or psychomimetic symptoms. Thus, while the patient is on high-dose propofol, it may be reasonable to use a ketamine dose at the higher end (0.2-0.3 mg/kg/hr).
advantages of dissociative-dose ketamine sedation
- Ketamine has direct bronchodilatory effects on the lungs.
- Ketamine tends to increase the blood pressure.
- Ketamine allows avoidance of toxicity due to other sedatives and analgesics.
disadvantages of dissociative-dose ketamine sedation
- (1) Concerns exist with regard to causing ICU delirium.
- (2) Lack of high-quality evidence:
- Given the rarity of truly refractory asthma, it's nearly impossible to perform an RCT.
- A before-after study demonstrated impressive improvements in blood gas parameters and chest mechanics, as shown below.(17301376) A similar study in pediatric patients showed nearly identical results, supporting this concept.(8905436)
potential indications for dissociative-dose ketamine
- There is insufficient high-level evidence to know exactly where this should fit in the treatment rubric. Situations where this should be considered are the following:
- (1) Refractory status asthmaticus with inability to ventilate safely.
- (2) Problematic hypotension due to conventional sedatives (e.g., propofol).
basics: how to provide dissociative-dose ketamine sedation in asthma:
- (1) Ketamine dosing:
- Load with 1-2 mg/kg bolus.
- Infusion at 1-4 mg/kg/hour. (35218742)
- (2) Other sedatives and analgesics can generally be stopped. (However, a propofol infusion may still be needed if there is problematic tachypnea that is causing gas trapping.)
- (3) Co-administration with an anticholinergic agent (e.g. glycopyrrolate 0.2 mg IV q6hr or atropine) may prevent problems with salivation and bronchorrhea.(25759905; 17301376, 8905436, 24082612)
- Note that using a systemic anticholinergic itself could theoretically promote bronchodilation.
Paralysis will make patients look beautiful (perfectly synchronous with the ventilator), but it carries substantial risks (delirium due to the depth of sedation required; myopathy). Some patients will require paralysis, but it should be avoided as much as possible.
how to avoid paralysis
- Respirolytic sedation 📖 may be used to suppress the patient's respiratory drive and thereby render the patient passive on the ventilator. If the patient is passive on the ventilator, there's really no added benefit to paralysis.
- Tailor the ventilator to minimize dyssynchrony. For example, use of pressure control mode may help reduce flow dyssynchrony (pressure control allows the patient to drive their own flow rate, which may be more comfortable).
- Permissive hypercapnia: 📖 Remember that the goal is merely to keep the patient safe on the ventilator, not to normalize the pH. Paralysis may improve the pH slightly, but this improvement usually isn't worth the iatrogenic harm from paralysis.
- Reducing trigger sensitivity? For a deeply sedated patient, reducing the trigger sensitivity is one way to reduce tachypnea and dynamic hyperinflation. This could be achieved simply by decreasing the PEEP to 5 cm in some patients with significant autoPEEP (discussed above 📖). This is a highly controversial concept that is not evidence-based.
if paralysis is needed, minimize iatrogenic harm
- The duration of paralysis should be limited to the shortest period achievable.
- (a) Rather than immediately initiating a paralytic infusion, try giving a bolus of paralytic and re-assessing the patient over time. Some patients may settle following a bolus of vecuronium, rather than requiring ongoing an ongoing paralytic infusion.
- (b) If a paralytic infusion is used: every day there should be a strong effort to lift paralytics. The odds of developing myopathy increase substantially with each additional day of paralysis.(10378560)
- Optimize the safety of paralytic infusions:
- Cisatracurium carries a lower risk of myopathy than aminosteroid paralytics (e.g., vecuronium).(23062076)
- Titrate the paralytic to the lowest dose that allows for ventilator synchrony.
ECMO for severe asthma
- ECMO offers the ability to improve CO2 clearance. However, patients nearly never die from hypercapnia. Therefore, the vast majority of patients with asthma should be manageable without ECMO. In the pre-ECMO era, asthma patients didn't die from hypercapnia and overall had very good outcomes.(18773325; 22188845)
- ECMO may be life-saving in complex patients with multiple coexisting problems (e.g. asthma complicated by a large bronchopleural fistula).
potential indications for ECMO?
- (1) Inability to perform deep permissive hypercapnia:
- Active neurologic disease with elevated intracranial pressure.
- Severe pulmonary hypertension or right ventricular failure.
- Pregnancy.
- (2) Inability to oxygenate:
- Profoundly severe asthma (possibly due to mucus plugging).
- Asthma plus bronchopleural fistula with large air leak.
- Asthma plus pneumonia or ARDS.
potential problems with inhalational anesthetics
- The main problem is that they generally require administration through an anesthesia ventilator. Such ventilators are generally not very sophisticated, and are unfamiliar to the ICU and respiratory therapy teams. Very simply, anesthesia circuits are not designed for prolonged use and they're not great at this. Use of an anesthesia circuit may lead to a host of problems:
- Anesthesia circuits aren't designed for continuous albuterol nebulization, so this may be impossible.
- Anesthesia circuits often have limited ability to perform advanced modes of ventilation.
- Alarm settings are typically unfamiliar, which may lead to delayed detection of complications (e.g. pneumothorax).
- Anesthesia circuits require continuous maintenance with use of beads to absorb CO2. Failure to change out the CO2 absorber frequently enough will cause CO2 rebreathing. (28612677)
- The anesthesiology circuit will typically need to be managed by an anesthesiologist. This may increase the number of people involved in caring for the patient, with attendant communication issues.
- Hypotension.
- Limits to the duration of inhalational anesthesia.
bottom line on inhalation anesthetics via an anesthesia circuit
- 🛑 There isn't enough evidence of benefit in order to justify using this treatment.
- Case series in the pediatric literature suggest that isoflurane decreases pCO2 by ~17 mm and improves pH by ~0.11 units within two hours. These improvements aren't that substantial – and probably don't justify the risks incurred by using the anesthesia circuit.(16614808)
Follow us on iTunes
The Podcast Episode
Want to Download the Episode?
Right Click Here and Choose Save-As
To keep this page small and fast, questions & discussion about this post can be found on another page here.

- Assumption that an asthma patient has asthma (rather than, for example, pneumothorax or pneumonia).
- Inadequate use of permissive hypercapnia for the ventilated asthmatic. Don't sweat the hypercapnia; trying to bring down the PaCO2 is often more harmful than helpful.
- Over-bagging of the asthmatic after intubation. This carries a high risk of gas trapping and pneumothorax. It's probably ideal to connect these patients to a ventilator as soon as possible to provide controlled ventilation.
- Note that “wheeze” or stridor you can hear across the room isn't due to asthma (suggests upper airway obstruction instead).
Guide to emoji hyperlinks 
= Link to online calculator.
= Link to Medscape monograph about a drug.
= Link to IBCC section about a drug.
= Link to IBCC section covering that topic.
= Link to FOAMed site with related information.
= Link to supplemental media.
References
- 03392363 Schroeckenstein DC, Bush RK, Chervinsky P, Busse WW. Twelve-hour bronchodilation in asthma with a single aerosol dose of the anticholinergic compound glycopyrrolate. J Allergy Clin Immunol. 1988 Jul;82(1):115-9. doi: 10.1016/0091-6749(88)90060-7 [PubMed]
- 03619169 Slovis CM, Daniels GM, Wharton DR. Intravenous use of glycopyrrolate in acute respiratory distress due to bronchospastic pulmonary disease. Ann Emerg Med. 1987 Aug;16(8):898-900. doi: 10.1016/s0196-0644(87)80530-9 [PubMed]
- 03792086 Walker FB 4th, Kaiser DL, Kowal MB, Suratt PM. Prolonged effect of inhaled glycopyrrolate in asthma. Chest. 1987 Jan;91(1):49-51. doi: 10.1378/chest.91.1.49 [PubMed]
- 07663804 Lougheed DM, Webb KA, O'Donnell DE. Breathlessness during induced lung hyperinflation in asthma: the role of the inspiratory threshold load. Am J Respir Crit Care Med. 1995 Sep;152(3):911-20. doi: 10.1164/ajrccm.152.3.7663804 [PubMed]
- 07781346 Emerman CL, Cydulka RK. A randomized comparison of 100-mg vs 500-mg dose of methylprednisolone in the treatment of acute asthma. Chest. 1995 Jun;107(6):1559-63. doi: 10.1378/chest.107.6.1559 [PubMed]
- 07952641 Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med. 1994 Dec;150(6 Pt 1):1722-37. doi: 10.1164/ajrccm.150.6.7952641 [PubMed]
- 08424280 Conti G, Dell'Utri D, Vilardi V, De Blasi RA, Pelaia P, Antonelli M, Bufi M, Rosa G, Gasparetto A. Propofol induces bronchodilation in mechanically ventilated chronic obstructive pulmonary disease (COPD) patients. Acta Anaesthesiol Scand. 1993 Jan;37(1):105-9. doi: 10.1111/j.1399-6576.1993.tb03609.x [PubMed]
- 08669670 Eames WO, Rooke GA, Wu RS, Bishop MJ. Comparison of the effects of etomidate, propofol, and thiopental on respiratory resistance after tracheal intubation. Anesthesiology. 1996 Jun;84(6):1307-11. doi: 10.1097/00000542-199606000-00005 [PubMed]
- 08905436 Youssef-Ahmed MZ, Silver P, Nimkoff L, Sagy M. Continuous infusion of ketamine in mechanically ventilated children with refractory bronchospasm. Intensive Care Med. 1996 Sep;22(9):972-6. doi: 10.1007/BF02044126 [PubMed]
- 10378560 Behbehani NA, Al-Mane F, D'yachkova Y, Paré P, FitzGerald JM. Myopathy following mechanical ventilation for acute severe asthma: the role of muscle relaxants and corticosteroids. Chest. 1999 Jun;115(6):1627-31. doi: 10.1378/chest.115.6.1627 [PubMed]
- 14739811 Groeben H, Mitzner W, Brown RH. Effects of the alpha2-adrenoceptor agonist dexmedetomidine on bronchoconstriction in dogs. Anesthesiology. 2004 Feb;100(2):359-63. doi: 10.1097/00000542-200402000-00026 [PubMed]
- 15539716 Silverman RA, Nowak RM, Korenblat PE, Skobeloff E, Chen Y, Bonuccelli CM, Miller CJ, Simonson SG. Zafirlukast treatment for acute asthma: evaluation in a randomized, double-blind, multicenter trial. Chest. 2004 Nov;126(5):1480-9. doi: 10.1378/chest.126.5.1480 [PubMed]
- 16236844 Hansel TT, Neighbour H, Erin EM, Tan AJ, Tennant RC, Maus JG, Barnes PJ. Glycopyrrolate causes prolonged bronchoprotection and bronchodilatation in patients with asthma. Chest. 2005 Oct;128(4):1974-9. doi: 10.1378/chest.128.4.1974 [PubMed]
- 16614808 Shankar V, Churchwell KB, Deshpande JK. Isoflurane therapy for severe refractory status asthmaticus in children. Intensive Care Med. 2006 Jun;32(6):927-33. doi: 10.1007/s00134-006-0163-0 [PubMed]
- 17301376 Heshmati F, Zeinali MB, Noroozinia H, Abbacivash R, Mahoori A. Use of ketamine in severe status asthmaticus in intensive care unit. Iran J Allergy Asthma Immunol. 2003 Dec;2(4):175-80 [PubMed]
- 18773325 Kao CC, Jain S, Guntupalli KK, Bandi V. Mechanical ventilation for asthma: a 10-year experience. J Asthma. 2008 Sep;45(7):552-6. doi: 10.1080/02770900801999090 [PubMed]
- 20085924 Mannam P, Siegel MD. Analytic review: management of life-threatening asthma in adults. J Intensive Care Med. 2010 Jan-Feb;25(1):3-15. doi: 10.1177/0885066609350866 [PubMed]
- 20956393 Ramsay CF, Pearson D, Mildenhall S, Wilson AM. Oral montelukast in acute asthma exacerbations: a randomised, double-blind, placebo-controlled trial. Thorax. 2011 Jan;66(1):7-11. doi: 10.1136/thx.2010.135038 [PubMed]
- 21597111 Perrin K, Wijesinghe M, Healy B, Wadsworth K, Bowditch R, Bibby S, Baker T, Weatherall M, Beasley R. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax. 2011 Nov;66(11):937-41. doi: 10.1136/thx.2010.155259 [PubMed]
- 22188845 Peters JI, Stupka JE, Singh H, Rossrucker J, Angel LF, Melo J, Levine SM. Status asthmaticus in the medical intensive care unit: a 30-year experience. Respir Med. 2012 Mar;106(3):344-8. doi: 10.1016/j.rmed.2011.11.015 [PubMed]
- 2225951 Gilman MJ, Meyer L, Carter J, Slovis C. Comparison of aerosolized glycopyrrolate and metaproterenol in acute asthma. Chest. 1990 Nov;98(5):1095-8. doi: 10.1378/chest.98.5.1095 [PubMed]
- 23062076 Price D, Kenyon NJ, Stollenwerk N. A fresh look at paralytics in the critically ill: real promise and real concern. Ann Intensive Care. 2012 Oct 12;2(1):43. doi: 10.1186/2110-5820-2-43 [PubMed]
- 24082612 Goyal S, Agrawal A. Ketamine in status asthmaticus: A review. Indian J Crit Care Med. 2013 May;17(3):154-61. doi: 10.4103/0972-5229.117048 [PubMed]
- 26716866 Lee SH, Kim N, Lee CY, Ban MG, Oh YJ. Effects of dexmedetomidine on oxygenation and lung mechanics in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery: A randomised double-blinded trial. Eur J Anaesthesiol. 2016 Apr;33(4):275-82. doi: 10.1097/EJA.0000000000000405 [PubMed]
- 24731521 Goodacre S, Cohen J, Bradburn M, Stevens J, Gray A, Benger J, Coats T; 3Mg Research Team. The 3Mg trial: a randomised controlled trial of intravenous or nebulised magnesium sulphate versus placebo in adults with acute severe asthma. Health Technol Assess. 2014 Apr;18(22):1-168. doi: 10.3310/hta18220 [PubMed]
- 25164315 Singhi S, Grover S, Bansal A, Chopra K. Randomised comparison of intravenous magnesium sulphate, terbutaline and aminophylline for children with acute severe asthma. Acta Paediatr. 2014 Dec;103(12):1301-6. doi: 10.1111/apa.12780 [PubMed]
- 25699177 Longrois D, Conti G, Mantz J, Faltlhauser A, Aantaa R, Tonner P. Sedation in non-invasive ventilation: do we know what to do (and why)? Multidiscip Respir Med. 2014 Nov 4;9(1):56. doi: 10.1186/2049-6958-9-56 [PubMed]
- 25759905 Schivo M, Phan C, Louie S, Harper RW. Critical asthma syndrome in the ICU. Clin Rev Allergy Immunol. 2015 Feb;48(1):31-44 [PubMed]
- 26033128 Leatherman J. Mechanical ventilation for severe asthma. Chest. 2015 Jun;147(6):1671-1680. doi: 10.1378/chest.14-1733 [PubMed]
- 26164393 Matsumoto T, Tomii K, Tachikawa R, Otsuka K, Nagata K, Otsuka K, Nakagawa A, Mishima M, Chin K. Role of sedation for agitated patients undergoing noninvasive ventilation: clinical practice in a tertiary referral hospital. BMC Pulm Med. 2015 Jul 13;15:71. doi: 10.1186/s12890-015-0072-5 [PubMed]
- 28612677 Shutes B, Frazier WJ, Tobias JD. An Unusual Complication With the Administration of a Volatile Anesthetic Agent for Status Asthmaticus in the Pediatric Intensive Care Unit: Case Report and Review of the Literature. J Intensive Care Med. 2017 Jul;32(6):400-404. doi: 10.1177/0885066617713169 [PubMed]
- 29105540 Laher AE, Buchanan SK. Mechanically Ventilating the Severe Asthmatic. J Intensive Care Med. 2018 Sep;33(9):491-501. doi: 10.1177/0885066617740079 [PubMed]
- 29131536 Bond KR, Horsley CA, Williams AB. Non-invasive ventilation use in status asthmaticus: 16 years of experience in a tertiary intensive care. Emerg Med Australas. 2018 Apr;30(2):187-192. doi: 10.1111/1742-6723.12876 [PubMed]
- 30633478 Stojak BJ, Halajian E, Guthmann RA, Nashelsky J. Intravenous Magnesium Sulfate for Acute Asthma Exacerbations. Am Fam Physician. 2019 Jan 15;99(2):127-128 [PubMed]
- 30996631 Miller A, VanHart DA, Gentile MA. Noninvasive ventilation in life-threatening asthma: A case series. Can J Respir Ther. 2017 Summer;53(3):33-36 [PubMed]
- 31443563 Kostakou E, Kaniaris E, Filiou E, Vasileiadis I, Katsaounou P, Tzortzaki E, Koulouris N, Koutsoukou A, Rovina N. Acute Severe Asthma in Adolescent and Adult Patients: Current Perspectives on Assessment and Management. J Clin Med. 2019 Aug 22;8(9):1283. doi: 10.3390/jcm8091283 [PubMed]
- 31690381 Agnihotri NT, Saltoun C. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2019 Nov 1;40(6):406-409. doi: 10.2500/aap.2019.40.4258 [PubMed]
- 32340824 Shaker MS, Oppenheimer J, Grayson M, Stukus D, Hartog N, Hsieh EWY, Rider N, Dutmer CM, Vander Leek TK, Kim H, Chan ES, Mack D, Ellis AK, Lang D, Lieberman J, Fleischer D, Golden DBK, Wallace D, Portnoy J, Mosnaim G, Greenhawt M. Reply to “Subcutaneous terbutaline as an alternative to aerosolized albuterol”. J Allergy Clin Immunol Pract. 2020 Jul-Aug;8(7):2450-2452. doi: 10.1016/j.jaip.2020.04.016 [PubMed]
- 35218742 Garner O, Ramey JS, Hanania NA. Management of Life-Threatening Asthma: Severe Asthma Series. Chest. 2022 Oct;162(4):747-756. doi: 10.1016/j.chest.2022.02.029 [PubMed]






