 CONTENTS
CONTENTS
- Rapid Reference 🚀
- Introduction: An emerging standard of care
- Physiology
- Clinical background
- Clinical utility
- Podcast
- Questions & discussion
- Pitfalls

Waveform capnography is emerging as a standard monitoring tool to improve safety among intubated patients. Failure to use waveform capnography contributed to >70% of ICU-related airway deaths in the NAP4 audit. Capnography was pioneered in the operating room, but the safety implications for all critically ill patients are clear (the standard of safety monitoring in the ICU shouldn't be lower than in the operating room). Capnography is increasingly recommended both to confirm endotracheal tube insertion and to subsequently monitor the patency and effectiveness of ventilation throughout the duration of intubation. Within the next decade, continuous waveform capnography will likely become a universal standard of care across all well-resourced intensive care units.
As the use of waveform capnography expands, we need to be thoughtful about integrating this into our practice. This involves several components:
- Paying attention to etCO2 values (e.g., noting them daily in reviews of the patient, along with other vital signs).
- Understanding changes in etCO2 within the context of other data (especially trends in minute ventilation).
- Understanding how to interpret etCO2 waveforms.
- Appreciating limitations of etCO2.

what is end-tidal CO2 (etCO2)?
- etCO2 is a measurement of the partial pressure of CO2 in gas expired at the end of exhalation (when exhaled gas will most closely resemble the alveolar CO2 concentration).
- Waveform capnography should be monitored in all intubated patients and displayed on the monitor (as above).
- Both the numeric value and the shape of the etCO2 tracing are important. If the etCO2 curve doesn't reach a plateau, then the numeric value is less reliable.
understanding dead space
- Dead space refers to inhaled gas that doesn't participate in carbon dioxide clearance. Three general sources of dead space are:
- Anatomic dead space (e.g., trachea and large bronchi) – with each breath gas enters these areas, but doesn't participate in gas exchange.
- Instrument dead space – for intubated patients, this includes any tubing interposed between the patient's airway and the site of fresh gas insufflation.
- Alveolar dead space – dysfunctional alveoli which receive ventilation but don't exchange CO2 well (or at all).
- Anatomic and instrument dead space are generally fixed. However, if the patient is ventilated with small tidal volumes, then a greater fraction of each breath will be wasted on dead space ventilation. Consequently, a strategy of high-frequency, low-tidal volume breaths will tend to achieve less CO2 clearance for any specific total minute ventilation.
- Alveolar dead space may be increased in most types of lung disease (reflecting dysfunction at the alveolar, vascular, or airway level). For example, increased dead space is seen in pulmonary embolism, in pneumonia or other parenchymal lung diseases such as aspiration, or in obstructive lung diseases such as asthma. The amount of alveolar dead space may change over time, as lung disease improves or deteriorates.
the relationship between etCO2 and arterial CO2 (PaCO2)

- The best way to conceptualize this is to imagine gas flowing from a high-functioning alveolus into the ventilator.
- #1) Within the high-functioning alveolus, the CO2 pressure will be equal to the arterial CO2 pressure.
- #2) As gas flows from this alveolus out of the lung, it will be diluted by dead space gas that will have a lower CO2 concentration (since this dead space gas doesn't absorb CO2 from the blood).
- #3) By the time gas reaches the endotracheal tube, the end-tidal CO2 concentration will be lower than the arterial CO2 tension.
- This leads to two foundational principles of etCO2:
- (1) Arterial CO2 should be higher than etCO2. Extraordinarily rarely, etCO2 can be slightly higher than arterial CO2 in pregnant patients with otherwise normal lungs.🌊 For the purpose of everyday critical care practice, it's reasonable to assume that the PaCO2 is above the etCO2.
- (2) The gap between the etCO2 and the PaCO2 is a reflection of the amount of dead space. Any increase in dead space (e.g., due to severely injured lungs), will widen the gap. In patients with normal lungs, the gap is typically ~3-10 mm. For patients with severe lung disease, the gap can be much greater.

Two general principles can guide us here:
#1) at steady state, cardiac output does not affect etCO2
- In an equilibrium state, the amount of CO2 produced by the body tissues must be cleared by the lungs. (26447854) Therefore,
- (a) The amount of CO2 clearance in the lungs will always equal the CO2 production by the body.
- (b) The concentration of CO2 in exhaled gas depends only on the body's CO2 production and the volume of gas exhaled per minute (the minute ventilation).
- (c) Cardiac output doesn't affect end-tidal CO2.
- Clinically, this is actually helpful because it simplifies matters. For a patient who is at steady state, the etCO2 reflects solely metabolic parameters (i.e., CO2 production by the body) and respiratory parameters (i.e., dead space and minute ventilation). At steady state, cardiac output does not affect etCO2.
- This is convenient, because if equilibrium etCO2 reflected respiratory and cardiac and metabolic parameters then it would become incredibly confusing.
- For stable patients on a ventilator, cardiac output should not affect the etCO2.
#2) dynamic changes in cardiac output can cause transient changes in etCO2
- Rapid changes in cardiac output can cause transient fluctuations in etCO2.
- To illustrate this, let's imagine what happens if the cardiac output suddenly drops from 5 liters/minute to 3 liters/minute:
- Immediately, there would be a reduction in CO2 delivery to the lungs. This would drop the etCO2.
- Over time, CO2 would build up in the tissues. This would increase the venous blood CO2 tension, eventually elevating etCO2 to its prior level.
- After re-equilibration, the drop in cardiac output would have no lasting impact on etCO2.
- Examples of changes in cardiac output that affect etCO2 include the following:
- Cardiopulmonary resuscitation is an example of a disequilibrium state, where etCO2 is strongly driven by cardiac output. Thus, higher-quality CPR will improve the cardiac output and increase the etCO2. Return of spontaneous circulation will cause an abrupt jump in etCO2. (more on this below)
- A passive leg raise maneuver may cause a transient increase in cardiac output among patients who are preload-responsive. This will cause a transient increase in etCO2. (more on this below)
The accuracy of etCO2 in predicting PaCO2 depends on how well the patient's lungs are functioning. Thus, different studies often reach opposite conclusions regarding the usefulness of etCO2 measurement. Studies involving patients with lung disease tend to conclude that etCO2 is inadequate to determine the PaCO2. Alternatively, studies involving patient populations with normal lung function will tend to conclude that etCO2 is sufficient to determine the patient's PaCO2. Overall, most studies of patients without lung disease show that PaCO2 is typically ~3-5 mm above the etCO2, and nearly always within <10-15 mm above the etCO2.(28993038, 27601718)
Even in stable patients on mechanical ventilation, PaCO2 normally fluctuates randomly with a standard deviation of +/- 3 mm CO2.(1583545, 6407807, 8020270, 19091262) Therefore, the PaCO2 itself is an inherently unstable parameter. A patient may have a certain PaCO2 for one moment, then they may wake up and breathe more and drop their PaCO2. Ongoing variation in PaCO2 naturally over time suggests that it's futile to try to be extremely precise about PaCO2 values. An additional reason not to worry about the exact PaCO2 values in most patients is that nobody knows the optimal PaCO2 values for intubated patients (e.g., some authors suggest that hypercapnia may be lung-protective and thus beneficial).(27060535)
etCO2 is less accurate in monitoring patients with lung disease (with increasing inaccuracy in the sickest patients). Alterations in ventilator settings may change the amount of dead space and the gap between etCO2 and PaCO2 (e.g., increasing the respiratory rate could lead to autoPEEP which causes some alveoli to be inadequately perfused; these non-perfused alveoli function as dead space). Thus, etCO2 cannot be assumed to track perfectly with PaCO2 in the sickest patients with respiratory failure.(26447854) etCO2 may nonetheless be worth trending in such patients, as substantive changes may reflect some sort of alteration in the patient's condition which bears further investigation.
We've obtained thousands of blood gas measurements on intubated patients in order to adjust their ventilators. Why do we do this? We were trained to do so. We are expected to do so. Yet, there is actually no high-quality evidence to support this practice. The lack of evidence doesn't mean that we shouldn't ever check blood gas parameters. However, lack of evidence should temper our zeal in pursuing this monitoring. In particular, let's consider the following points.
#1) There's no evidence to support routine blood gas monitoring among intubated patients.
- There has never been a high-quality RCT comparing monitoring of blood gas parameters versus no monitoring of blood gas parameters among intubated patients.
- Monitoring blood gas values involves expense, pain, and blood loss. However, this practice has never been subjected to rigorous investigation to determine the appropriate intensity of blood gas monitoring. This may be analogous to the practice of routine chest X-ray measurement in the ICU – a practice which was assumed to be essential for decades, until it was proven that it actually wasn't.
- A study comparing routine blood gas measurement versus clinician-triggered blood gas measurement (to answer a specific question) is needed.
#2) The target PaCO2 for intubated patients is generally unknown.
- Aside from a few specific situations (e.g., intracranial pressure elevation), very little is known about the target PaCO2 among intubated patients.
- It's widely assumed that targeting “normal” blood gas values will improve outcomes, but available evidence suggests that this is actually wrong. Specifically, the ARDS literature demonstrates that most patients can tolerate respiratory acidosis perfectly well (permissive hypercapnia) – and trying to drive down PaCO2 to “normal” levels may actually cause iatrogenic harm.
- Some data suggests that hypercapnia may be lung-protective. Therefore, it's possible that for some patients, hypercapnia could be desirable.
- Overall, for most intubated patients, there's little persuasive evidence that routine adjustments in the ventilator will improve patient-centered outcomes.
So, what PaCO2 values should we target? We shouldn't throw out the baby with the bathwater – some amount of control over PaCO2 is probably sensible. In the absence of high-quality data, the following targets might be reasonable.
a) patients requiring tighter control over PaCO2
- Some patients may deserve closer monitoring, perhaps the following groups:
- (1) Neuro-critically ill patients with elevated intracranial pressure.
- (2) Patients with right heart failure due to pulmonary hypertension (hypercapnia and acidosis increase pulmonary vascular resistance).
- (3) Pregnant patients.
- Targeting relatively “normal” PaCO2 values in these patients (e.g., 35-45 mm) seems reasonable.
- Occasional, other patients with specific pathologies may require specific pH management strategies (e.g., severe salicylate intoxication patients undergoing alkalinization).
b) patients who don't require tight control over PaCO2
- Among most other intubated patients, it's probably unnecessary to target such a tight range of PaCO2 values.
- The optimal strategy for titrating PaCO2 in these patients is unknown, so any goals will by definition be arbitrary.
- One reasonable strategy might be to adjust PaCO2 to target a pH of roughly 7.2-7.5. The rationale for this is as follows:
- pH seems to be a more important physiological variable than PaCO2. Normal physiology seems to be designed to defend the pH more robustly than defending the PaCO2 (e.g., respiratory compensation for metabolic acid/base disorders demonstrates that evolution cares more about pH than PaCO2). Enzyme and protein function depends on pH, not PaCO2 – explaining why pH is more important.
- Alkalemia is generally not well tolerated, but 7.50 represents only a very mild degree of alkalemia.
- Acidemia is generally tolerated far better than alkalemia. The lower extent of a “safe” pH is unknown and may vary between patients. However, 7.2 seems to be well within a safe range (e.g., as established by common practices surrounding permissive hypercapnia).
- For patients with pulmonary disease (e.g., ARDS, severe asthma, bronchopleural fistula), permissive hypercapnia might be better (e.g., targeting a pH > 7.15 or 7.10). However, a pH target of 7.2-7.5 may be reasonable for most intubated patients.
Aside from simply paying attention to the etCO2 value, it is essential to also inspect the waveform. At the most basic level, the normalcy of the waveform informs about the accuracy of the etCO2 value (if the waveform is abnormal or erratic, then the etCO2 value is less reliable). At the next level, abnormal waveforms may suggest specific pathologies.
normal etCO2 waveform

- Key aspects:
- (1) Relatively sharp upstroke, flat top that reaches a plateau, and rapid downstroke.
- (2) etCO2 values are reasonably stable from breath to breath (they may vary by a few mm, but they shouldn't be all over the place).
airway obstruction etCO2 waveform (Fin-shaped)

- Key aspects:
- (1) Mild obstruction will cause an increase in the slope of the top of the waveform.
- (2) Severe obstruction may cause the entire waveform to resemble a shark fin.
- Differential diagnosis:
- Small airway obstruction (e.g., COPD or asthma).
- Larger airway obstruction (partially obstructed airway).
- Why does exhaled CO2 rise over time due to obstruction?
- Early in expiration, relatively unobstructed alveoli empty. Gas spends less time in these alveoli, so the pCO2 is lower.
- Later on during, the more obstructed alveoli start emptying. Gas stays longer in these alveoli, so their pCO2 is higher.
- A fin-shaped capnograph suggests that the etCO2 may be substantially lower than the blood pCO2.
patient efforts

- Key aspects:
- Small divots are seen in the etCO2 pattern.
- These occur synchronously with patient effort.
- Significance:
- (1) At the most basic level, these reveal that the patient isn't paralyzed.
- (2) Notches occurring during expiration represent “missed triggers”: the patient is trying to trigger another breath, but failing to do so. This may be a sign of autoPEEP or an incorrectly set trigger sensitivity. Notches can also be a sign of flow starvation if the ventilator is set in a volume-cycled mode.
elevated baseline
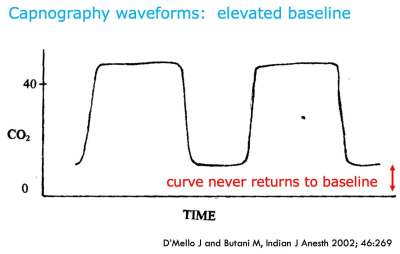
- Key aspects: etCO2 tracing never returns to zero baseline.
- Significance: Patient is rebreathing CO2. This may result from a faulty expiratory valve, or (in an anesthetic machine) saturation of the CO2 system.
waveform capnography is the gold-standard test to confirm initial endotracheal tube placement
- Esophageal placement may generate a brief etCO2 tracing, but this will rapidly be extinguished within about six breaths. This initial etCO2 signal may be caused by carbon dioxide which has been insufflated into the gastrointestinal tract during bag-mask ventilation.
- Potential problems:
- (1) Capnography can fail if tubing is kinked (for side-stream capnography) or is connected incorrectly to the monitor. These problems may be avoided by testing the etCO2 sensor prior to intubation (by placing it close to the patient's mouth, where it should register some CO2 production). Alternatively, portable etCO2 sensors have the advantage of requiring no tubing or connections with the monitor.
- (2) Endotracheal tube placement in a right mainstem bronchus will reveal a normal etCO2 tracing.
- (3) Endotracheal tube placement above the vocal cords in the glottis may reveal a near-normal appearing etCO2 tracing.
- Absence of an etCO2 tracing should be interpreted to mean that the endotracheal tube is in the esophagus and requires replacement (“No Trace Wrong Place”). Note that pulse oximetry may take minutes to drop, so a normal saturation should not be reassuring if there is no etCO2 return. Even patients in cardiac arrest should have an etCO2 waveform (albeit with a low etCO2 value).
waveform capnography to monitor endotracheal tube placement over time
- Capnography may serve as a safety feature, alerting providers to tube dislodgement or displacement.
- Note, however, that an adequate etCO2 waveform does not guarantee that the endotracheal tube is in the trachea.
- 🛑 If the endotracheal tube is lying above the vocal cords, a normal-appearing waveform may still be visible if the patient is spontaneously breathing!
Waveform capnography is useful for many aspects of cardiac arrest management. Current ACLS guidelines recommend incorporation of quantitative etCO2 capnography. (26472989)
endotracheal tube placement confirmation
- Capnography should be used to confirm the position of the endotracheal tube, as in any patient.
- Capnography may be especially useful in cardiac arrest patients, as other conventional markers of endotracheal tube placement may be less reliable in these patients (e.g., auscultation is impossible during cardiopulmonary resuscitation, and low CO2 production may make qualitative paper indicators of CO2 difficult to interpret).
quality of CPR
- etCO2 reveals the amount of CO2 being cleared from the body, which is a reflection of the quality of CPR, as well as the body's CO2 production.
- etCO2 values may be used to coach the resuscitation team on the quality of CPR being produced.(28993038)
- etCO2 <10 mm suggests that an improved technique or a different provider should be attempted.
- etCO2 >20 mm is desired.
prognostication
- Low etCO2 may be due to suboptimal CPR technique, but it may also be due to death (cessation of cellular respiration, leading to a lack of CO2 production).
- Most studies have evaluated etCO2 after 20 minutes of CPR:
- etCO2 <10 mm suggests low likelihood of survival.
- etCO2 10-20 mm may represent a grey zone.
- etCO2 >20 mm suggests higher likelihood of survival.
- Whether or not to continue resuscitative efforts should not be based solely on etCO2 values. However, this may provide some supplemental, objective information to help inform on this decision (e.g., a combination of cardiac standstill and etCO2 < 10 mm suggests futility).
early detection of return of spontaneous circulation (ROSC)
- Return of circulation will often cause an abrupt jump in the etCO2 value.
- A rapid increase in etCO2 by >10 mm is highly suggestive of ROSC.(28993038)
Immediately after intubation, etCO2 can help adjust the ventilator settings. Even if you are going to check a blood gas, it still makes sense to begin by adjusting the ventilator based on etCO2 (and subsequently checking your work using a blood gas). Immediately adjusting the ventilator based on etCO2 may accelerate achieving a target pH and thereby avoid the requirement for multiple blood gas measurements.
for a patient with critical neurologic disease
- In this context it's often desirable to target a low-normal PaCO2 (to avoid cerebral vasodilation or vasoconstriction).
- Following intubation, adjust the ventilator to target an etCO2 of ~30 mm. This will generally result in a PaCO2 within the normal range (35-45 mm). Then check a blood gas, to ensure that the pCO2 is actually within a safe range.
for patients without critical neurologic disease
- For most patients, targeting a pH of 7.2-7.5 may be reasonable (discussed further above).
- If we know the patient's baseline bicarbonate value, then we can use this to establish an initial etCO2 target (table below).
- The etCO2 target is determined by calculating the PaCO2 which would give the patient a pH of 7.5.
- In reality, the PaCO2 will be greater than the etCO2. This will place the patient's pH into a safe range (7.2-7.5). For most patients, the gap between etCO2 and PaCO2 will be ~5-10 mm, which will leave them at the higher end of this range (e.g., a pH of ~7.4). Patients with lung disease and a larger etCO2-PaCO2 gap, may have a somewhat higher PaCO2 and a thus a lower pH (e.g., a pH of ~7.3) – but their pH will still likely lie within a safe range.
- Targeting an etCO2 immediately, based on the patient's baseline bicarbonate, should rapidly bring the patient to a safe pH range (thereby avoiding common mistakes, such as post-intubation alkalemia in a patient with chronic hypoventilation and elevated bicarbonate). If necessary, further fine-tuning may be based on the results of blood gas analysis.


etCO2-only strategy
- Monitoring CO2 clearance based solely on etCO2 (without ever checking a blood gas) may be considered if the following conditions are met:
- (#1) etCO2 is expected to be reasonably accurate:
- Good etCO2 waveform (e.g., reaches a reasonably flat plateau, etCO2 values remain stable from breath to breath).
- No known lung disease (e.g., chest X-ray shows reasonably normal lungs).
- Absence of substantial hypoxemia.
- (#2) Precise CO2 control isn't necessary:
- No elevation of intracranial pressure.
- No right ventricular failure due to pulmonary hypertension.
- Not pregnant.
- (#3) Absence of a severe metabolic pH abnormality.
- (#4) etCO2 target can be achieved with a relatively normal minute ventilation (e.g., roughly 6-10 liters/minute).
examples of etCO2-only monitoring
- Monitoring of ventilation using only etCO2 is common practice among anesthesiologists in the operating room (e.g., for patients undergoing general anesthesia without substantive physiological derangement). Unfortunately, this concept hasn't yet been fully translated into general critical care practice.
- Another example would be a patient intubated for airway protection due to inebriation. There is no reason to demand that their pH is precisely perfect. The patient will wake up and be extubated in the morning. It's irrelevant whether the patient's pH is 7.25, 7.35, or 7.45 (provided that the patient is hemodynamically stable and otherwise doing well).
etCO2 trending combined with occasional blood gas management
- Most patients in an ICU will not meet the criteria above for etCO2-only monitoring. For such patients, it may be sensible to occasionally obtain a blood gas measurement to determine the pCO2-etCO2 gap. Even if blood gasses are measured intermittently, etCO2 remains useful for continuous tracking of the patient's physiology.
minute ventilation
- This refers to the total volume of gas which is inhaled by the patient per minute. This is an extremely important parameter which is continuously displayed on the ventilator.
- A normal minute ventilation may be around ~6-8 liters/minute. Minute ventilation can be affected by a number of factors, including:
- Larger patients will require higher minute ventilation.
- More metabolically active patients (e.g., fever, shivering) will require higher minute ventilation.
- Pathologically increased dead space (e.g., ARDS, asthma) will increase minute ventilation requirement.
- Anxiety or pain may cause patients to hyperventilate, increasing their minute ventilation to more than is required (potentially causing hypocarbia).
- Very low tidal volumes may increase the minute ventilation requirement, because a higher fraction of each breath is being wasted on ventilating anatomic dead space (i.e., the trachea and large airways).
- Patients who are awake and triggering the ventilator will often regulate their own minute ventilation to achieve a normal PaCO2. For example, if their lung function deteriorates with worsening dead space, they will naturally increase minute ventilation to achieve a stable PaCO2. One exception, of course, is that opioids and sedatives (especially propofol) reduce the respiratory drive, so patients on these may not correctly regulate their own CO2.
- Minute ventilation is a hugely important parameter which doesn't receive the attention that it deserves. For example:
- Low minute ventilation (e.g., <6 liters/minute) suggests hypoventilation and hypercapnia.
- An acute rise in minute ventilation may indicate acutely deteriorating lung function (e.g., pulmonary embolism or pneumonia).
integration of etCO2 and minute ventilation
- Normally, minute ventilation and etCO2 should be inversely related:
- Hypoventilation (reduced minute ventilation) causes an increase in etCO2.
- Hyperventilation (excess minute ventilation) causes a decrease in etCO2.
- Thus: if etCO2 and minute ventilation change in opposite directions, this suggests hypoventilation or hyperventilation.
- If etCO2 and minute ventilation don't change in opposite directions, this suggests that something else is going on. Possible causes for etCO2 to change independently from changes in minute ventilation are shown below:

why detection of hypercapnia is important during spontaneous breathing trials
- A spontaneous breathing trial involves placing a patient on minimal ventilatory support to assess their ability to support their own work of breathing. The most commonly utilized parameters used to identify failure of a spontaneous breathing trial include tachypnea, air hunger, and rapid/shallow breathing index. All of these parameters rely on the assumption that the patient's respiratory drive is functioning normally (so that the patient will strive to achieve a normal PaCO2).
- These commonly monitored parameters may fail to detect patients with a poor respiratory drive, who may develop occult hypoventilation during a spontaneous breathing trial (without distress or tachypnea). This can occur in patients with chronic hypercapnia or patients on medications that suppress the respiratory drive, who have blunted responses to hypercapnia.
detection of occult hypercapnia during spontaneous breathing trials
- Hypercapnia evolving during a spontaneous breathing trial can be measured using serial blood gas measurements. (14585115) However, this is laborious and overall low-yield (due to the low rate of occult hypercapnia).(15507165) Consequently, the practice of performing serial blood gas measurement during spontaneous breathing trials has largely disappeared.
- Occult hypercapnia still does rarely occur though, so this shouldn't be ignored entirely. etCO2 is a convenient and useful tool to ensure that patients are continuing to ventilate adequately during a spontaneous breathing trial. If the etCO2 increases by >10 mm during a spontaneous breathing trial, this should raise some concern about the development of hypercapnia.(8796386)
- However, a pathological rise of etCO2 must be differentiated from the rise seen in patients who are being over-ventilated prior to the spontaneous breathing trial (in which case, a rise in pCO2 may simply represent normal physiology).
the concept of fluid responsiveness
- Fluid responsiveness refers to the ability of additional volume to transiently increase the cardiac output. Unfortunately, the concept is fraught with hazard, because most administered fluid will not remain in the vasculature. Thus, administering fluid to a patient with fluid responsiveness often will fail to cause any sustained improvement in cardiac output.
- The main utility of fluid responsiveness is identifying that patients are not fluid responsive, because this argues strongly that they will not benefit from additional volume administration.
- The fact that a patient is fluid responsive does not indicate that they will benefit from fluid!
- People should normally be fluid responsive. Lack of fluid responsiveness is a pathological condition (e.g., due to volume overload, cardiac dysfunction, or both).
use of fluid responsiveness as a guide to deresuscitation?
- An alternative use of fluid responsiveness might be to guide deresuscitation of the patient with systemic volume overload.
- Absence of fluid responsiveness is a pathological state, which may be caused by systemic congestion. In this context, absence of fluid responsiveness could be used as a clue that the patient could benefit from ongoing diuresis.
- If fluid administration doesn't affect cardiac output, it stands to reason that fluid removal wouldn't affect cardiac output either. This may signal that diuresis is safe.
using etCO2 to determine fluid responsiveness during passive leg raise

- Passive leg raise involves transiently raising the legs to cause an auto-bolus of fluid. This may be used to determine whether the patient is volume responsive.
- The Achilles heel of the passive leg raise is that it requires the ability to rapidly measure small changes in cardiac output. This can be done with Doppler echocardiography, but rapidly obtaining reliable measurements is difficult.
- End-tidal CO2 may be useful here, as an easily and immediately measurable index of changes in cardiac output. An increase in etCO2 by >5% appears to have reasonable sensitivity (71%-91%) and specificity (94%-100%) for fluid responsiveness in two studies of patients breathing passively on the ventilator. (22990869, 22449292)
- One study found that volumetric capnography has superior performance compared to etCO2.(23182383) Volumetric capnography integrates the total volume of CO2 being excreted over time (as the area under the capnograph/time curve). Volumetric capnography would be expected to be the most accurate measurement technique, as it is most directly tied to cardiac output. Especially among patients who are breathing spontaneously with variable tidal volume duration, volumetric capnography would be expected to outperform etCO2. Volumetric capnography is already incorporated in many ventilators and will likely play a growing role in the future of ICU capnography.
Follow us on iTunes
To keep this page small and fast, questions & discussion about this post can be found on another page here.
- Quantitative waveform capnography should be continuously monitored among all intubated patients. This is a safety measure that should be incorporated into protocols and assessments by respiratory therapists, nurses, and physicians.
- We should be trending and paying attention to minute ventilation and etCO2 values in all intubated patients (in the same way that we track other vital signs). Changes in these values may provide early signals of clinical deterioration.
- etCO2 may be used to reduce the number of blood gasses which are measured, leading to reduced blood loss and pain. In patients with normal lung function, etCO2 might be able to replace blood gas monitoring entirely.
References
- 08796386 Saura P, Blanch L, Lucangelo U, Fernández R, Mestre J, Artigas A. Use of capnography to detect hypercapnic episodes during weaning from mechanical ventilation. Intensive Care Med. 1996;22(5):374-381. doi:10.1007/BF01712151 [PubMed]
- 14585115 Salam A, Smina M, Gada P, et al. The effect of arterial blood gas values on extubation decisions. Respir Care. 2003;48(11):1033-1037. [PubMed]
- 15507165 Pawson SR, DePriest JL. Are blood gases necessary in mechanically ventilated patients who have successfully completed a spontaneous breathing trial?. Respir Care. 2004;49(11):1316-1319. [PubMed]
- 15636648 Thompson JE, Jaffe MB. Capnographic waveforms in the mechanically ventilated patient. Respir Care. 2005 Jan;50(1):100-8. [PubMed]
- 22449292 Monge García MI, Gil Cano A, Gracia Romero M, Monterroso Pintado R, Pérez Madueño V, Díaz Monrové JC. Non-invasive assessment of fluid responsiveness by changes in partial end-tidal CO2 pressure during a passive leg-raising maneuver. Ann Intensive Care. 2012;2:9. Published 2012 Mar 26. doi:10.1186/2110-5820-2-9 [PubMed]
- 22990869 Monnet X, Bataille A, Magalhaes E, et al. End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med. 2013;39(1):93-100. doi:10.1007/s00134-012-2693-y [PubMed]
- 23182383 Young A, Marik PE, Sibole S, Grooms D, Levitov A. Changes in end-tidal carbon dioxide and volumetric carbon dioxide as predictors of volume responsiveness in hemodynamically unstable patients. J Cardiothorac Vasc Anesth. 2013;27(4):681-684. doi:10.1053/j.jvca.2012.09.025 [PubMed]
- 23221867 Kodali BS. Capnography outside the operating rooms. Anesthesiology. 2013 Jan;118(1):192-201. doi: 10.1097/ALN.0b013e318278c8b6 [PubMed]
- 26447854 Nassar BS, Schmidt GA. Capnography During Critical Illness. Chest. 2016;149(2):576-585. doi:10.1378/chest.15-1369 [PubMed]
- 26472989 Neumar RW, Shuster M, Callaway CW, et al. Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S315-S367. doi:10.1161/CIR.0000000000000252 [PubMed]
- 27601718 Siobal MS. Monitoring Exhaled Carbon Dioxide. Respir Care. 2016 Oct;61(10):1397-416. doi: 10.4187/respcare.04919 [PubMed]
- 28993038 Long B, Koyfman A, Vivirito MA. Capnography in the Emergency Department: A Review of Uses, Waveforms, and Limitations. J Emerg Med. 2017;53(6):829-842. doi:10.1016/j.jemermed.2017.08.026 [PubMed]



