CONTENTS
Clinical aspects
- Rapid Reference 🚀
- Bradycardia is dangerous: physiology review
- Causes of bradycardia
- General evaluation for the etiology
ECG evaluation & subtypes of bradycardia
- Heart blocks
- Sinus node abnormality
- Irregularity & clumped beating
General approach to treatment
- Overall strategy
- Medical treatments
- Electrical treatments
 the effect of tachycardia on cardiac output is often overestimated
the effect of tachycardia on cardiac output is often overestimated
- Tachycardia has a mixed impact on cardiac output:
- Increase in heart rate tends to increase the cardiac output.
- Decreased filling time tends to decrease the stroke volume, which decreases cardiac output.
- Mild-moderate tachycardia will generally increase cardiac output. This is a normal physiologic response to stress. The effect of increased heart rate predominates here.
- Severe tachycardia (heart rates >>150 b/m) may drop the cardiac output because the heart doesn't have time to fill with blood during diastole, causing a reduced stroke volume.
- The deleterious effect of heart rate on cardiac output is often overestimated. For example, if a patient has atrial fibrillation with a heart rate of 150 b/m, it's unlikely that cardioversion or rate control will improve cardiac output. Usually, slowing down a moderate tachycardia will cause deterioration.
the effect of bradycardia on cardiac output is often underestimated
- Bradycardia directly pulls down the cardiac output, potentially causing shock.
- Slowing down the heart rate may cause a minimal increase in diastolic filling, thereby increasing the stroke volume. However, this compensatory factor is weak and extremely limited. For example, if the heart rate decreases by a factor of two, the stroke volume cannot possibly double!
- In severe bradycardia, the cardiac output must be low. This is simple math.
- Cardiogenic shock is defined as inadequate cardiac output to support organ function. Some patients can compensate for low cardiac output without developing shock. However, with increasingly severe bradycardia, there should be an increasing concern for cardiogenic shock.
don't be fooled by normal-pressure bradycardia

- “the heart rate is 30 b/m but the blood pressure is fine… I think we can send her to the floor”
- Some patients with bradycardia will maintain a normal blood pressure, due to an endogenous sympathetic response causing vasoconstriction. Despite a normal blood pressure, these patients still have a low cardiac output and still may be in shock.
- Rare patients can present with severe bradycardia and severe hypertension (#3 in figure above). Hypertension is caused by a massive sympathetic response, as the body struggles to compensate for the bradycardia. This dangerous situation must be managed thoughtfully, because the sympathetic response is actually keeping the patient alive. Aggressive vasodilation to treat the “hypertensive emergency” may cause hemodynamic collapse. Management should focus on correction of the bradycardia. Once the heart rate normalizes, the endogenous sympathetic response should relax and everything will resolve.
progressive bradycardia is often a harbinger of death
- Progressively worsening bradycardia is often seen immediately preceding death (“the patient is bradying down”).
- If the patient's heart rate is consistently dropping in front of your eyes don't just stand there – get some epinephrine. Fast.
- The differential diagnosis of bradycardia here is broader than usual and may include such entities as severe hypoxemia and right ventricular failure from massive PE. Immediate evaluation should focus on the ABCs: airway, breathing, and circulation (bedside echocardiogram).
one more reason to fear bradycardia: torsade de pointes
- Torsade de pointes is a pause-dependent arrhythmia, which is more likely to occur at slower heart rates. Furthermore, bradycardia itself may prolong the QT interval. (15974204, 21151381) It's possible that leaving patients in a severely bradycardic state may increase their risk of torsade.
sinus node dysfunction
medications
- Alpha-2 agonists (clonidine, guanfacine, tizanidine, dexmedetomidine).
- Alpha-blockers (e.g. prazosin).
- Cholinergic agent.
- Beta-blockers.
- Diltiazem, verapamil.
- Digoxin.
- Antiarrhythmics:
- Frequently worsens mild sinus node disease: flecainide, propafenone, sotalol.
- Infrequently worsens mild sinus node disease: digoxin, quinidine, procainamide, disopyramide, moricizine.
- Rarely worsens mild sinus node disease: lidocaine, phenytoin, mexiletine, tocainide. (Gaggin 2021)
- Antiseizure medications (carbamazepine, lacosamide, phenytoin).
- Other:
- Antihistamines.
- Antidepressants.
- Donepezil.
- Lidocaine.
- Lithium.
- Phenothiazines (rarely).
- Propofol infusion syndrome.
- Ropinirole.
- Ticagrelor.
metabolic
- Hyperkalemia 📖 (especially BRASH syndrome 📖).
- Hypermagnesemia.
- Hypothyroidism or hyperthyroidism.
- Hyperparathyroidism.
- Hypothermia.
- Severe hypoxia / hypercapnia / acidemia (sinus bradycardia is a common pathway of impending death from any cause).
MI
- Inferior MI with involvement of the sinoatrial node (usually due to proximal involvement of the right coronary artery).
- Myocarditis.
other
- Vagal stimulation.
- Elevated intracranial pressure (ICP).
- Rheumatologic disorders (lupus, systemic sclerosis).
- Infiltrative disorders (hemochromatosis, amyloidosis).
- Genetic disorders (e.g., Duchenne muscular dystrophy).
- Iatrogenic:
- Cardiac surgery, trauma.
- Electrophysiological procedures.
- Idiopathic/senile sinus node dysfunction (usually occurs in elderly patients with idiopathic degeneration of the sinus node). (O'Keefe 2021)
AV block (dysfunction of the AV node and/or conduction system)
medications
- Alpha-2 agonists (clonidine, tizanidine, dexmedetomidine).
- Antiarrhythmics.
- Antiseizure medications (carbamazepine, lacosamide).
- Beta-blockers.
- Nondihydropyridine calcium channel blockers (verapamil, diltiazem).
- Digoxin (often associated with accelerated junctional rhythm).
- Miscellaneous:
- Amphotericin B.
- Bortezomib.
- Clonazepam.
- Cisplatin.
- Cyclophosphamide.
- Lidocaine.
- Omeprazole.
- Pregabalin.
- Ticagrelor.
- Trazodone.
metabolic
- Hypothyroidism or hyperthyroidism.
- Hypophosphatemia.
- Hypoglycemia.
- Hyperkalemia:
- Isolated hyperkalemia (only profound).
- BRASH syndrome.
MI
- Heart block s/p MI: 📖
- Inferior MI with AV node ischemia or elevated vagal tone.
- Anterior MI causing conduction blocks.
infection
- Lyme disease.
- Syphilis.
- Aortic valve endocarditis with ring abscess.
- Myocarditis.
- Viral infections (including CMV, influenza H1N1).
inflammation
- Rheumatoid arthritis, lupus, systemic sclerosis, polymyositis, dermatomyositis.
- Granulomatosis with polyangiitis.
other
- Infiltrative disease (e.g., amyloidosis, hemochromatosis).
- Hereditary neuromuscular diseases (e.g., myotonic dystrophy).
- Takotsubo cardiomyopathy.
- Iatrogenic/traumatic:
- Cardiac surgery.
- Electrophysiological procedures.
- Transcutaneous aortic valve replacement.
- Transcatheter closure of ventricular septal defect.
- Pulmonary artery catheter placement.
- Alcohol septal ablation for hypertrophic cardiomyopathy.
- Myocardial contusion.
- Congenital third-degree heart block.
- Idiopathic fibrosclerosis of the conduction system:
- Lev disease is more common, seen in elderly.
- Lenegre disease may occur in younger patients.
[#1/3] ECG: focus on three things
- Rhythm diagnosis (e.g., sinus bradycardia vs. heart block).
- Signs of hyperkalemia (e.g., peaked T-waves).
- Signs of ischemia.
[#2/3] medication review
- The list of potentially causative medications is in the section above. ☝️
- Points of particular interest:
- Active medication list?
- Recent medication changes, including dose titration?
- Drug interactions?
- Renally cleared meds plus acute kidney injury?
[#3/3] bradycardia investigation package
- Chemistries including Ca & Mg, glucose.
- TSH (thyroid stimulating hormone).
- Lyme serology (patients can get lyme year round in may locales).
- Troponin (if MI suggested by history/ECG).
- Digoxin level (for patients taking digoxin).
- Echocardiogram (evaluate for valvular disease, infiltrative disorders, tumors, etc.).
ECG criteria for 1° degree AV block
- PR >200 ms (5 boxes).
- No dropped beats.
- Constant PR intervals.
trying to sort out the etiology
- (1) Duration of QRS – perhaps most important aspect.
- Block below the AV node (in His-Purkinje system) almost always accompanied by wide QRS.
- Trifascicular block is a combination of 1st degree AV block plus RBBB plus hemiblock (either right or left anterior hemiblock). This increases risk of progression to complete heart block.
- (2) Degree of PR prolongation:
- If PR >240 ms, this strongly suggests block within the AV node (abnormal his-purkinje conduction seldom prolongs conduction >200 ms).
causes include
- Medications:
- AV node:
- Beta-blockers.
- Non-dihydropyridine calcium channel blockers (diltiazem, verapamil).
- Digoxin.
- Below AV node:
- 1A antiarrhythmics (e.g. disopyramide, procainamide, quinidine).
- 1C antiarrhythmics (e.g. flecainide).
- AV node:
- Electrolyte disturbances (hyperkalemia).
- Ischemic heart disease (e.g., inferior MI).
- Myocarditis.
- Intrinsic AV nodal disease.
- Lyme disease.
- Infiltrative diseases (e.g., amyloidosis, sarcoidosis).
- Increased vagal tone (e.g. sleep, athletes).
- Idiopathic (PR rarely >280 ms in normal people).
clinical significance
- Isolated first-degree block is usually benign. This may be seen in ~0.7% of normal adults. (Gaggin 2021)
- Block below the AV node (e.g., with wide QRS) is more likely to progress to complete heart block (especially trifascicular block, as discussed above).
Mobitz I (aka Wenckebach block) is generally a benign phenomenon caused by mismatch between heart rate and AV node sympathetic tone.
diagnostic criterion for Mobitz I
- (1) Progressive increase in PR until QRS is dropped.
- If the P-waves can't be seen, then it may be noticed that the R-R interval becomes progressively shortened until a beat is dropped.
- (2) If sinus node is >135, then Mobitz I is adaptive (“2:1 conduction”).
causes of Mobitz I
- Inferior MI with AV nodal ischemia.
- Myocarditis.
- Mediations:
- Digoxin.
- Beta-blocker.
- Calcium channel blocker.
- Clonidine.
- Lithium.
- Flecainide, sotalol, propafenone. (O'Keefe 2021)
- Pacer.
- Lyme disease.
- Normal individuals:
- Physiologically high vagal tone during sleep.
- Athletes.
ECG findings:
- ECG criteria:
- PR interval is fixed.
- No evidence of atrial prematurity.
- Mobitz II is often associated with infranodal conduction disease on ECG.
ECG differential diagnosis
- Early PACs which are nonconducted (blocked PACs).
Mobitz II is caused by conduction system dysfunction, for example:
- MI: Anteroseptal infarction.
- Endocarditis.
- Valvular or congenital heart disease.
- Medications (e.g., procainamide, disopyramide, quinidine).
2:1 block be Mobitz I (AV nodal block) or Mobitz II (infranodal block). Mobitz I is more benign, whereas Mobitz II has a higher risk of progression to higher degrees of AV block (and may be an indication for pacemaker placement).
ECG features favoring Mobitz I:
- Sinus node rate >135 suggests Mobitz I (blocking can be adaptive rather than pathologic).
- PR prolongation
- Intermittent 3:2 block, or other ratios of Mobitz I conduction
- QRS has a narrow complex.
- Association with inferior MI.
ECG features of Mobitz II:
- QRS has a widened complex.
- History of syncope.
- Association with anterior MI. (O'Keefe 2021)
response to exercise, atropine, or beta-agonists (e.g., isoproterenol or epinephrine)
- Improves Mobitz I (by stimulating AV node).
- Will make Mobitz II look worse (speed up the P-wave speed, will exaggerate the severity of the block).
diagnostic criteria
- [1] Atrial rate > Ventricular rate.
- [2] Ventricular rhythm maintained by junctional or ventricular escape rhythm with slow rate (<45 b/m).
- [3] Independent atrial and ventricular activities (AV dissociation).
- [4] Enough P-waves to probe all phases of the cardiac cycle (no conduction ever).
differential diagnosis
- High-degree Mobitz II can look a lot like 3rd degree block (clinically this difference may not matter).
ventriculophasic sinus arrhythmia
- Seen in ~30% of 3rd degree block, less often with high-degree 2nd degree block.
- PP interval containing the QRS complex is shorter than the PP interval without a QRS complex.
- This will be confusing, because it may slightly alter the regular PP intervals.
causes of 3rd degree block
- These are listed above: ⚡️
main ECG features
- RR interval of escape rhythm:
- Constant (<40 ms variation)
- Rate is <60 (usually ~40-55).
- QRS should be narrow (unless there is an underlying conduction abnormality).
- 💡 Differentiating a ventricular escape rhythm versus junctional escape with aberrancy may be based on similar principles that are utilized to differentiate ventricular tachycardia versus supraventricular tachycardia with aberrancy. 📖
- Retrograde P-waves
- May precede, be superimposed on, or follow QRS complex depending on the site within the AV node that generates the impulse.
- P before QRS: P-QRS interval is usually <110 ms (2.75 mm).
- P after QRS: QRS-P interval may be 200 ms or longer
- May precede, be superimposed on, or follow QRS complex depending on the site within the AV node that generates the impulse.
differential diagnosis
- Accelerated junctional rhythm 📖 (heart rate is >60).
- Ectopic atrial escape rhythm: if inverted P-waves are seen >110 ms (>2.75 mm) before the QRS complex.
clinical significance
- Junctional rhythms are generally reliable and capable of achieving an adequate rate to support perfusion.
ECG criteria: requires all of the following
- Rate typically 30-40 b/m (can range over 20-50 beats/minute).
- QRS abnormal and wide (although foci near the septum can have near-normal QRS complexes).
- Rate of supraventricular impulse arriving at the ventricle is slower than the ectopic ventricular pacemaker.
differential diagnosis
- (1) Hyperkalemia may cause a wide-complex bradycardic rhythm with invisible P-waves.
- (2) Junctional escape rhythm with pre-existing conduction disease.
- 💡 Differentiating a ventricular escape rhythm versus junctional escape with aberrancy may be based on similar principles that are utilized to differentiate ventricular tachycardia versus supraventricular tachycardia with aberrancy (e.g., capture or fusion beats may help confirm the presence of a ventricular rhythm). 📖
- (3) If the heart rate is >> 50 b/m, then this may be accelerated idioventricular rhythm (AIVR).
clinical significance
- ⚠️ Ventricular escape rate is NOT a dependable rate. Patients are at risk for progression to asystole.
- Unless the cause may be reversed immediately, a backup pacemaker should be placed (e.g. temporary transvenous pacemaker).
definition
- Sinus bradycardia is defined as HR <60 with normal P-wave morphology.
- However, sinus bradycardia usually isn't clinically significant unless the heart rate is <50. In well-conditioned healthy people, the heart rate may drop even lower during sleep.
differential diagnosis
- If rate <40, consider 2:1 sinoatrial exit block
- Presence of sinus arrhythmia (small variations in P-P interval) may support hyperactive vagal activity
causes of sinus bradycardia
- Medications:
- Beta-blockers (including amiodarone).
- Diltiazem, verapamil.
- Sympatholytics (e.g. clonidine, dexmedetomidine).
- Parasympathomimetics (e.g. neostigmine).
- Digoxin.
- Antiarrhythmics (IA, IB, IC).
- Lithium.
- Methadone.
- Metabolic disturbances:
- Severe hypoxemia / hypercapnia / acidemia (impending death).
- Uremia.
- Advanced liver disease.
- Hyperkalemia or hypokalemia.
- Propofol infusion syndrome.
- Hypothyroidism.
- Hypothermia.
- Neurological disorders:
- Cushing reaction due to elevated intracranial pressure.
- Neurogenic shock.
- Sinus node dysfunction:
- Chronic degenerative changes.
- Infiltrative disease (sarcoidosis, amyloidosis, hemochromatosis).
- Collagen vascular disease (SLE, RA, scleroderma).
- Surgical trauma.
- Myocarditis, pericarditis.
- Inferior MI with involvement or the right coronary artery.
- Vagally mediated:
- Transient (carotid sinus syndrome, vomiting/coughing/straining/micturition, vasovagal syncope).
- Glaucoma.
- Neck abnormality with lymphadenopathy or tumor.
- Trained athletes with high resting vagal tone.
- Acute hypertension.
evaluation of cause
- Labs: Electrolytes (including Ca/Mg/Phos), TSH
- Medication review
- Neurologic examination if relevent
manifestations of sinus node dysfunction
overview of manifestations of sinus node dysfunction
- Sinus arrest.
- Sinus bradycardia in the absence of another cause.
- Chronotropic incompetence.
- Tachy-brady syndrome: sinus node dysfunction is often combined with atrial tachyarrhythmias.
- AF with slow ventricular response.
sinus pause/arrest
- ECG findings:
- PP interval (pause) >2 seconds.
- Not a multiple of the baseline P-P interval (that's sinoatrial exit block).
- ECG differential diagnosis:
- Suppression by PACs (look for PAC preceding the dropped beat, it may be buried in the T-wave). Blocked PACs are the most common cause of a pause. (O'Keefe 2021)
- Sinoatrial block (long cycle = multiples of the baseline PP interval).
- Marked sinus arrhythmia.
- Overdrive suppression after ectopic tachycardia.
- Clinical significance of sinus pause/arrest?
- Length:
- Pauses <3 seconds are commonly seen in healthy people and rarely symptomatic
- Pauses >3 seconds and not occurring during sleep are often pathologic and may cause symptoms. This can be a criterion for pacemaker placement in the context of sick sinus syndrome.
- Occasionally seen in normal people with increased vagal tone or hypersensitive carotid sinus. Other causes of sinus node disease are listed below.
- Length:
sinoatrial exit block
- ECG findings:
- Impulse from the sinus node to the atrium is blocked.
- Mobitz I: Shortening of PP interval, followed by a PP pause that is less than twice the normal PP interval
- Mobitz II: Pause is near multiple of the normal PP interval
- ECG differential diagnosis: Atrial bigeminy.
- Clinical significance:
- May be seen in 1% of normal subjects. (Gaggin 2021)
- If there is symptomatic sinus node dysfunction, evaluation and treatment is outlined below.
causes & management
causes of sinus node dysfunction
- These are listed above. ⚡️
management of sinus node dysfunction
- Initial supportive care is similar to the treatment of bradycardia in general.
- Remove any treatable etiologies (primarily medication discontinuation, as appropriate).
- For persistent sinus node dysfunction, consider placement of a permanent pacemaker.
generators of irregularity
- (1) Primary generator
- Atrial fibrillation
- Multifocal atrial tachycardia (MAT)
- Frequent ectopic beats (PACs, PVCs, etc)
- Sinus irregularity (generally very mild)
- (2) Regular generator with irregular block
- AFlutter with variable block
- Focal atrial tachycardia with variable block
clumped beating (“regularly irregular”)
- PACs (atrial bigeminy, atrial trigeminy, etc.)
- Mobitz I or Mobitz II
 overview: algorithm for bradycardic peri-arrest
overview: algorithm for bradycardic peri-arrest
- Bradycardic peri-arrest may be loosely defined as severe bradycardia with marked shock and concern for immediate cardiac arrest. The algorithm below shows a maximally aggressive strategy designed to prevent further deterioration into cardiac arrest.
- There are two “arms” of therapy: electrical & medical.
- It's hard to predict which patients will respond best to medical or electrical therapy.
- Proceed simultaneously down both arms of therapy as rapidly as possible until the patient is stabilized.
- For patients with mild signs of organ malperfusion (e.g. normal blood pressure but poor urine output), then a more gradual and stepwise approach may be most appropriate. For example, simply starting an epinephrine infusion will often improve heart rate and perfusion.

🛑 when possible, tailor your therapy based on the underlying problem
- The following sections describe a general approach to bradycardia which is usually effective. However, this isn't the best approach for every specific type of bradycardia.
- Whenever possible, it's essential to try to figure out why the patient is bradycardic – and to address that specific problem.
problems with atropine
- At low doses, atropine may cause paradoxical bradycardia. (15114081, 25634857, 16115264, 12734175)
- Atropine works by poisoning the vagus nerve, so it is only effective for bradycardias mediated by excess vagal tone. It will predictably fail in cases of high-degree AV block.
- Atropine is contraindicated in patients who have had cardiac transplantation, in whom it may precipitate asystole. (15114081)
- Atropine may stabilize the patient for 30-60 minutes, but then wear off. This can initially make the patient appear stable, only to deteriorate later on (once everyone has stopped paying so much attention).
strategy when using atropine?
- If atropine is the most immediately available drug, then give it. Alternatively, if you have immediate access to epinephrine, it may be more effective to go straight to epinephrine.
- Atropine is traditionally the 1st-line medical therapy. However, for very unstable patients, epinephrine is more reliably effective and may be preferable.
- Start at 1 mg atropine, additional doses can be given to a maximal dose of ~3 mg (although larger doses may be needed in patients with cholinergic poisoning).
- Overall only ~25% of patients have a complete response to atropine, so don't delay other therapies while waiting for atropine to work. (10459592)
- Don't give atropine, sit back, and expect that it will fix everything. Give atropine while simultaneously preparing epinephrine and transcutaneous pacing – with the full expectation that the atropine will often fail.
advantages of epinephrine
- Epinephrine is available everywhere and can be obtained quickly.
- Unlike atropine, epinephrine stimulates the entire myocardium. This provides epinephrine with a broader spectrum of efficacy for various mechanisms of bradycardia. (18701603)
- Epinephrine is safe for peripheral infusion (you don't need to place a central line).🌊
epinephrine strategy
- Boluses for peri-arrest patient:
- For patient on verge of a cardiac arrest, bolus with doses of ~20-50 mcg epinephrine.
- Boluses will stabilize the patient for a few minutes, but this is only a temporary bridge to an epinephrine infusion.
- Epinephrine infusion:
- The usual dose is 2-10 mcg/min (but there is no hard upper limit in a crashing patient).
- Dosing strategy depends on how unstable the patient is. For more unstable patients, start high and down-titrate as the patient responds. For patients who are fairly stable, start low and gradually up-titrate.
- Figure out how to achieve this at your unit:
- a) If you have immediate access to pre-mixed epinephrine bags, know how to use them (know their concentration and how many ml's are needed to deliver push-dose epinephrine).
- b) If you don't have immediate access to pre-mixed epinephrine, then, read on…
the “dirty epi drip”
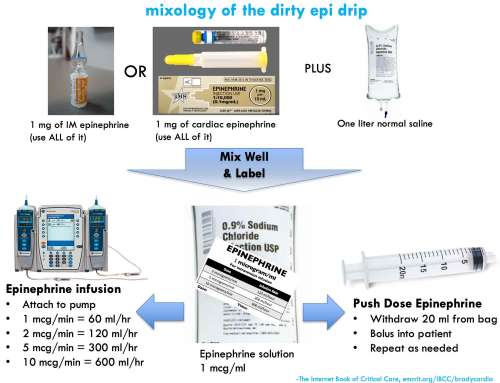 Mixing a bag of epinephrine is easy. This is conventionally termed a “dirty epi drip,” but if done properly it's a safe and precise way to deliver epinephrine.
Mixing a bag of epinephrine is easy. This is conventionally termed a “dirty epi drip,” but if done properly it's a safe and precise way to deliver epinephrine.
step #1: create the epinephrine reservoir bag
- Inject 1 mg of epinephrine into a liter bag of normal saline. One milligram of epinephrine can be obtained either from an entire syringe of cardiac epinephrine (1:10,000) or an entire vial of IM epinephrine (1:1000).
- Squish around the bag to mix well.
- Label the bag.
step #2: push dose epinephrine
- For a patient in peri-arrest, you will want to deliver small boluses of epinephrine until the patient stabilizes.
- Fill up an empty 20 cc syringe with diluted (1 mcg/ml) epinephrine from your one-liter bag.
- Bolus the patient with 20 ml of this solution, which will deliver a bolus of 20 mcg epinephrine.
- Refill your 20 ml syringe and repeat as needed.
- Push-dose epinephrine is a temporizing solution. As soon as the patient stabilizes, start an epinephrine infusion.
step #3: epinephrine infusion
- Attach your bag of epinephrine to an infusion pump and set the rate. For example:
- Infuse at 60 ml/hour to achieve 1 mcg/min infusion
- Infuse at 240 ml/hour to achieve 4 mcg/min infusion
- Infuse at 600 ml/hour to achieve 10 mcg/min infusion
advantages of the “dirty epi” bolus & drip strategy:
- This is relatively idiot-proof. As long as you mix well and label the bag, it should be pretty difficult to make dosing errors:
- Regardless of what type of epinephrine you use, you will be fine (either 1:1,000 or 1:10,000 will work).
- It's physically impossible to bolus a lethal dose of epinephrine after it's been diluted to 1 mcg/ml (you would need a >100 ml syringe, which doesn't exist).
- Even if you run the epinephrine bag in wide open, you would only be delivering about ~30 mcg/min of epinephrine – so again, it's basically impossible to deliver a lethally high epinephrine dose.
- Constructing a reservoir bag of epinephrine encourages a rapid transition from push-dose epinephrine to an epinephrine infusion (which is ultimately a safer and more controlled strategy).
- This is a viable approach during epinephrine shortages:
- Easily performed with 1:1000 epinephrine, if your shop runs out of 1:10,000 epinephrine.
- One vial of epinephrine can be used for both pushes & drip, thereby conserving medication.
Along with epinephrine, calcium is a drug which is often under-utilized in bradycardia. IV calcium is potentially effective for various etiologies listed below. Calcium is pretty safe (unless it extravasates), so when other therapies fail it makes sense to try to some calcium.
calcium-responsive bradycardias:
- Hyperkalemia.
- Hypocalcemia.
- Hypermagnesemia.
- Calcium-channel blocker.
- Beta-blocker (maybe).
dosing
- Bradycardia of unknown etiology: Try one round of calcium (1 gram calcium chloride or 3 grams calcium gluconate).
- Known or suspected hyperkalemia: Start with 1 gram of calcium chloride or 3 grams of calcium gluconate. If ineffective and patient is dangerously unstable, consider additional calcium. The maximal dose of calcium is unknown in this situation. Bedside chemistry monitoring with an iSTAT might be helpful here, shooting for moderate hypercalcemia (e.g., an ionized calcium level of 2-3 mM).
dobutamine
- Dobutamine is mostly a beta-agonist, with very weak alpha-adrenergic activity. Unlike epinephrine, dobutamine tends to cause systemic vasodilation:
- Dobutamine might be perfect for a patient with bradycardia and normal/elevated blood pressure, where you're trying to increase cardiac output (without increasing the blood pressure).
- Dobutamine isn't a good choice for the crashing, hypotensive patient. If the dobutamine fails to accelerate the heart rate then it could act solely as a vasodilator, and thereby cause worsening hypotension.
- Dobutamine might not be quite as safe for peripheral infusion as epinephrine. If you're giving dobutamine for prolonged peripheral infusion, monitor the site carefully and avoid any IVs in the hand or wrist.
isoproterenol
- This is an excellent drug for bradycardia if you can get ahold of it.
- Isoproterenol is a pure beta-agonist, which is safe for peripheral infusion. Isoproterenol does seem to be a bit more powerful than epinephrine (there are some patients who don't respond to epinephrine yet will respond to isoproterenol).
- The main drawbacks to isoproterenol are logistic. Isoproterenol is insanely expensive in the United States (an infusion may cost several thousand dollars). Many hospitals don't have it. Even if your hospital does have it, it will usually take time getting it from pharmacy.🌊
dopamine
- Dopamine has a long track record of use in symptomatic bradycardia. The main advantage of dopamine is that it's stable at room temperature, so it may be more widely available in pre-mixed bags (e.g., in ambulances).
- Disadvantages of dopamine compared to epinephrine:
- 1) Dopamine can cause skin necrosis with prolonged infusion.
- 2) At high doses, dopamine may act predominantly as a vasoconstrictor. This can be undesirable if you're mostly looking for chronotropy.
- If dopamine is the most readily available agent, then use it. When you have time, consider switching over to an epinephrine infusion.
aminophylline 💊
- May occasionally be useful in certain situations, including:(30586772, 31311698)
- The usual loading dose is 6 mg/kg ideal body weight, infused over 20-30 minutes. If effective, this may be followed by a maintenance infusion of aminophylline (e.g., 0.3-0.5 mg/kg/hour) which may eventually be transitioned to chronic oral maintenance theophylline. The optimal dosing of aminophylline/theophylline for bradycardia is unknown. Some case reports suggest that relatively low doses may be effective, but it may also be reasonable to target a theophylline level of ~10-20 ug/ml (similar to its use in obstructive airway disease). 📄 (18154483, 34316890)
advanced toxicologic therapies
- Local Anesthesia Systemic Toxicity (LAST) 📖
- Suspect in any bradycardic patient on lidocaine infusion or recently treated with nerve block.
- Front-line therapy is intralipid.
- Beta-blocker and/or calcium-channel blocker toxicity 📖
- Advanced toxicologic treatments are primarily useful for patients who present with massive overdose. However, these therapies can also be considered for patients with bradycardia due to therapeutic misadventures.
- Treatment may involve high-dose insulin, glucagon, or intralipid.
Transcutaneous pacing is often the fastest strategy to increase the heart rate. Even if it doesn't capture, the discomfort may be enough to trigger a sympathetic response that keeps the patient alive. Either way, this is a temporary measure until more definitive stabilization is possible (e.g., transvenous pacing).
 pad configuration
pad configuration
- Air is a poor conductor of electricity, so placing pads that overlie the lungs is a poor strategy.
- Anterior-posterior pad placement may be preferred (image above)(26849986)
- Anterior pad is on the left side of the lower part of the sternum, covering the “left parasternal window” of the heart. Based on experience with echocardiography, this is the most reliable site of contact between the heart and the soft tissue of the chest.
current
- If patient is crashing, start at maximal current and work your way down after the patient has stabilized.
- If patient is doing OK, then start low and titrate up.
- If the patient is doing OK, then you probably wouldn't really want to do transcutaneous pacing at all. However, it may be useful to determine if the patient responds to transcutaneous pacing. Proving that transcutaneous pacing will capture the heart may help you decide whether placing a transvenous pacemaker is necessary in a borderline patient.
- Continue pacing at 10-20 mA above the minimum energy required for capture.
- Usually ~40-80 mA required to achieve capture (possibly more in obesity or obstructive lung disease). (24044868)
beware of pseudo-pacing

- Pseudo-pacing is when the pacemaker isn't capturing the myocardium, but the monitor displays a heart rate equal to the transcutaneous pacemaker. This provides a false sense of security, because the monitor looks great.
- Always confirm that the pacemaker is capturing via one of the following methods:
- Pulse oximetry waveform shows a pulse matching the pacemaker (image above).
- Bedside echocardiogram confirms myocardial contraction with pacing.
- Pulse, preferably far away from the chest (e.g., femoral pulse or dorsalis pedis – to avoid being fooled by twitching of the chest musculature).
analgesia/sedation?
- This can be limited by patient's instability. Low-dose fentanyl and/or ketamine might be reasonable.
- Deep sedation & intubation to allow for tolerance of transcutaneous pacing is a popular approach, but probably not the best. The instability induced by sedation and intubation may outweigh benefits from transcutaneous pacing. Also, if the patient becomes hyperinflated on the ventilator, this could theoretically lead to loss of capture by the transcutaneous pacer.
Transvenous pacing is the most invasive strategy, but also the most effective (with success rates >95%). (17212976) Indications are roughly as follows:
- Unstable bradycardia which doesn't respond to other interventions (e.g., epinephrine).
- High-degree AV blocks that leave the patient at ongoing risk of deterioration (e.g., Mobitz II, third-degree heart block with wide-complex escape rhythm).
have a kit, know your kit, love your kit
- The unit should have everything needed for a transvenous pacer in one specific location (e.g., a large box or drawer in a resuscitation cart). This will include the transvenous pacemaker itself, the venous sheath, the pacing generator, wires, and adapter pins.
- Use the appropriate size venous sheath for the pacemaker:
- If you ask for a random venous sheath, you're likely to be given an 8.5 French sheath which is designed to accommodate a Swan-Ganz catheter. This sheath cannot be used for placement of a pacemaker wire – it will be too large (leading to leakage of blood out of the sheath or air embolization into the sheath). The pacer sheath will be smaller.
- The crux of this procedure is familiarity with the pacer kit stocked in your unit. Ideally the unit should have a non-sterile kit available for training purposes. In an emergency, the muscle memory for how all the parts get assembled will be invaluable.
- Also know how to work the pacing generator. Newer digital pacing generators are designed for electrophysiologists, so they can be confusing. Make sure you're familiar with your hospital's device.
 insertion site
insertion site
- In general, the sites which allow for most facile floating of the pacemaker are:
- 1st choice: Right internal jugular (straight shot into the RV).
- 2nd choice: Left subclavian (smooth arc through the larger vessels into the heart).
appropriate current while floating the wire
- Depending on how unstable the patient is, there are roughly two strategies for floating a temporary pacemaker:
- Honey Badger Mode: As you are floating the wire, increase the current to 20 mA. This will capture the heart as rapidly as possible, which is preferable if the patient is actively dying. The problem is that capture may occur while the wire is in the atrium, so this approach doesn't always result in ideal placement of the temporary pacemaker. The goal here is to stabilize the patient as soon as possible, you can fiddle with the pacemaker later.
- Usual technique: For a patient who isn't actively dying, float the pacemaker with a lower amplitude (e.g., 5 mA). This usually won't capture the myocardium until you're close to the right ventricular myocardium. This strategy is better for optimizing the ideal placement of the pacemaker. After you gain capture, advance the pacer a couple of millimeters further and deflate the balloon – this will often position it optimally, lying against the right ventricle.
ultrasound guidance
- This isn't necessary, but can be helpful. Ultrasound requires a second operator to reach under the sterile drape and position the ultrasound.
- A four-chamber view (e.g., subcostal 4-chamber) is generally best, as this can allow visualization of the wire entering the right atrium and ventricle.
- Potential value:
- (1) If you advance the pacer wire over ~30 cm and don't see it in the right atrium, then the wire has probably passed straight into the the inferior vena cava. Deflate the balloon, pull back to ~15 cm, and try floating again.
- (2) Ultrasound allows fine-tuning of the placement procedure. For example, once you're through the tricuspid valve you can slow down – you only have a few more centimeters left to go.
- (3) If you visualize the wire in the right ventricle but you're not getting capture then there might be a problem with the pacer box. Make sure all the wires are connected correctly and the settings are correct.
complications
- Most complication relate to placement of the pacemaker sheath in the vein (e.g., pneumothorax or bleeding).
- There is a very small, yet finite risk of hemopericardium (<0.6%) which can lead to tamponade 📖. (30543806) If a patient deteriorates following placement of a temporary transvenous pacemaker, ultrasonography should be performed to exclude hemopericardium.
Follow us on iTunes
The Podcast Episode
Want to Download the Episode?
Right Click Here and Choose Save-As
To keep this page small and fast, questions & discussion about this post can be found on another page here.
- Don't assume that because the blood pressure is normal, the patient is adequately perfusing and doing fine. Some patients vasoconstrict and maintain normal blood pressure, despite organ malperfusion.
- For an unstable patient, don't get fixated on any specific intervention. Continue working through a series of electrical and mechanical therapies until something works (figure below).
- Don't be afraid to use push-dose epinephrine and peripheral epinephrine infusions for an unstable patient.
- Don't forget to get a good medication history, focusing on recent medication changes and drugs which can accumulate in renal dysfunction (e.g. digoxin, atenolol).
- Don't be fooled by transcutaneous pacemaker pseudocapture. The fact that the chest is twitching and the monitor shows a normal heart rate means nothing – it's still possible that the myocardium isn't being captured.
- Remember that bradycardia can be caused by myocardial infarction and various intoxications – so fixing the heart rate may not be enough to fix the patient.
- Try to imagine every piece of your transvenous pacemaker kit and how they it is assembled. If you can't do this, you need practice with the kit. The most common procedural hang-up is being unfamiliar with the kit and pacemaker generator.
Guide to emoji hyperlinks 
= Link to online calculator.
= Link to Medscape monograph about a drug.
= Link to IBCC section about a drug.
= Link to IBCC section covering that topic.
= Link to FOAMed site with related information.
- 📄 = Link to open-access journal article.
= Link to supplemental media.
References
- 10459592 Brady WJ, Swart G, DeBehnke DJ, Ma OJ, Aufderheide TP. The efficacy of atropine in the treatment of hemodynamically unstable bradycardia and atrioventricular block: prehospital and emergency department considerations. Resuscitation. 1999 Jun;41(1):47-55. doi: 10.1016/s0300-9572(99)00032-5 [PubMed]
- 12734175 Maruyama K, Mochizuki N, Hara K. High-degree atrioventricular block after the administration of atropine for sinus arrest during anesthesia. Can J Anaesth. 2003 May;50(5):528-9. doi: 10.1007/BF03021079 [PubMed]
- 15114081 Bernheim A, Fatio R, Kiowski W, Weilenmann D, Rickli H, Brunner-La Rocca HP. Atropine often results in complete atrioventricular block or sinus arrest after cardiac transplantation: an unpredictable and dose-independent phenomenon. Transplantation. 2004 Apr 27;77(8):1181-5. doi: 10.1097/01.tp.0000122416.70287.d9 [PubMed]
- 15974204 Ashworth SW, Levsky ME, Marley CT, Kang CS. Bradycardia-associated torsade de pointes and the long-QT syndromes: a case report and review of the literature. Mil Med. 2005 May;170(5):381-6. doi: 10.7205/milmed.170.5.381 [PubMed]
- 16115264 Chin KJ, Seow SC. Atrioventricular conduction block induced by low-dose atropine. Anaesthesia. 2005 Sep;60(9):935-6. doi: 10.1111/j.1365-2044.2005.04346.x [PubMed]
- 17212976 Sodeck GH, Domanovits H, Meron G, Rauscha F, Losert H, Thalmann M, Vlcek M, Laggner AN. Compromising bradycardia: management in the emergency department. Resuscitation. 2007 Apr;73(1):96-102. doi: 10.1016/j.resuscitation.2006.08.006 [PubMed]
- 18154483 Whitman CB, Schroeder WS, Ploch PJ, Raghavendran K. Efficacy of aminophylline for treatment of recurrent symptomatic bradycardia after spinal cord injury. Pharmacotherapy. 2008 Jan;28(1):131-5. doi: 10.1592/phco.28.1.131 [PubMed]
- 18701603 Vavetsi S, Nikolaou N, Tsarouhas K, Lymperopoulos G, Kouzanidis I, Kafantaris I, Antonakopoulos A, Tsitsimpikou C, Kandylas J. Consecutive administration of atropine and isoproterenol for the evaluation of asymptomatic sinus bradycardia. Europace. 2008 Oct;10(10):1176-81. doi: 10.1093/europace/eun211 [PubMed]
- 21151381 Namboodiri N. Bradycardia-induced Torsade de Pointes – An arrhythmia Less Understood. Indian Pacing Electrophysiol J. 2010 Oct 31;10(10):435-8 [PubMed]
- 24044868 Deal N. Evaluation and management of bradydysrhythmias in the emergency department. Emerg Med Pract. 2013 Sep;15(9) [PubMed]
- 25456780 Cortes J, Hall B, Redden D. Profound symptomatic bradycardia requiring transvenous pacing after a single dose of tizanidine. J Emerg Med. 2015 Apr;48(4):458-60. doi: 10.1016/j.jemermed.2014.10.005 [PubMed]
- 25634857 Carron M, Veronese S. Atropine sulfate for treatment of bradycardia in a patient with morbid obesity: what may happen when you least expect it. BMJ Case Rep. 2015 Jan 29;2015:bcr2014207596. doi: 10.1136/bcr-2014-207596 [PubMed]
- 26849986 Seifert PC, Yang Z, Reines HD. Crisis Management of Unstable Bradycardia in the OR. AORN J. 2016 Feb;103(2):215-23. doi: 10.1016/j.aorn.2015.12.012 [PubMed]
- 27484658 Wung SF. Bradyarrhythmias: Clinical Presentation, Diagnosis, and Management. Crit Care Nurs Clin North Am. 2016 Sep;28(3):297-308. doi: 10.1016/j.cnc.2016.04.003 [PubMed]
- 30543806 Metkus TS, Schulman SP, Marine JE, Eid SM. Complications and Outcomes of Temporary Transvenous Pacing: An Analysis of > 360,000 Patients From the National Inpatient Sample. Chest. 2019 Apr;155(4):749-757. doi: 10.1016/j.chest.2018.11.026 [PubMed]
- 31311698 Sidhu S, Marine JE. Evaluating and managing bradycardia. Trends Cardiovasc Med. 2020 Jul;30(5):265-272. doi: 10.1016/j.tcm.2019.07.001 [PubMed]





