.
Introduction: Perpetual controversy
.
The use of magnesium for AF has been a controversial topic for decades. Magnesium is a normal electrolyte, so it is cheap and has an excellent safety profile. Ironically, this is also magnesium's Achilles heel, because this has caused the pharmaceutical industry to have no interest in it. This leaves us with relatively sparse clinical evidence.
.
Evidence for magnesium in atrial fibrillation
.
(1) Cardioversion
.
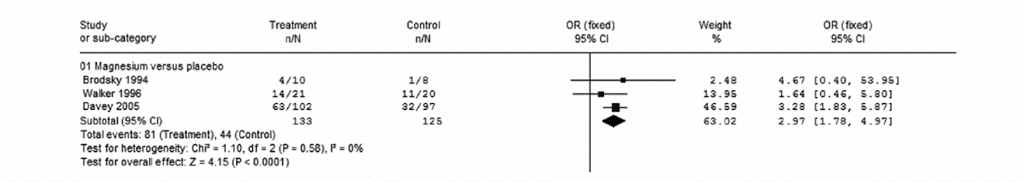
It is debatable whether magnesium alone causes cardioversion. Two meta-analyses published in 2007 reached opposite conclusions (Ho vs. Onalan). Overall Onalan et al. appears more reliable, as these authors were more discerning in the studies that they included (Ho et al. included many smaller studies and counted one study twice)(2). The results from Onalan et al. are shown below.
.
(2) Adjunctive agent for cardioversion
.
Even if magnesium alone fails to cause cardioversion, it may still improve the success of subsequent attempts at cardioversion, either electrical or medical. Adjunctive magnesium increases the likelihood of cardioversion due to class III anti-arrhythmic agents (e.g. ibutilide and dofetilide; Ganga 2013). Simultaneously, magnesium also reduces the risk of torsade de pointes, a significant problem with these agents. The combination of potassium and magnesium administration has been shown to improve the success rate of electrical cardioversion (Sultan 2012).
.
(3) Maintenance of sinus rhythm
.
Does magnesium help maintain sinus rhythm? Although indirect evidence, the highest quality data comes from studies regarding the prevention of postoperative atrial fibrillation. Magnesium has been shown in several RCTs and meta-analyses to reduce the incidence of postoperative atrial fibrillation by about 50% (Cochrane Review 2013).
.
(4) Improving rate control
.
Both the Onalan and Ho meta-analyses concur that magnesium improves rate control (e.g., figure from Onalan below). Although reductions in heart rate are statistically significant, the effect size is modest (e.g. average heart rate ~15 b/m lower than placebo group; Davey 2005, Walker 1996).
.
.
(5) Overall efficacy in AF
.
The likelihood of achieving either rate or rhythm control was higher with magnesium compared to placebo or calcium channel blocker (Onalan 2007):
.
.
(6) Safety
.
Compared to placebo or other treatments, magnesium showed a trend towards a reduced rate of major adverse effects (defined broadly as any event requiring additional intervention, treatment discontinuation, or considered significant by authors).
.
.
Magnesium pharmacology: The rationale for a continuous infusion
.
Less than 1% of the body's magnesium is located in the serum, with far greater amounts located within cells. Unfortunately, serum levels of magnesium may not reflect the intracellular magnesium stores. In particular, the serum magnesium level may be normal or slightly low despite substantial intracellular magnesium depletion. Magnesium deficiency is common in critical illness, where it is associated with mortality and arrhythmia (Tong 2005).
.
It is difficult to replete intracellular magnesium stores. This is commonly observed when trying to treat hypomagnesemia: a single intravenous dose may transiently increase the serum magnesium level, but the next day the magnesium level often hasn't improved much. There are two reasons for this. First, the total intracellular magnesium deficit may be much larger than the administered dose. Second, cells aren't great at absorbing magnesium, so much of the administered magnesium ends up getting excreted in the urine. For a patient with reasonable renal function, the only way to rapidly replete total body magnesium stores is with a continuous intravenous infusion (1).
.
Evidence: Magnesium infusions for critically ill patients with AF
.
There are two studies regarding magnesium infusions in critically ill AF patients.
.
Moran 1995
.
This was a prospective RCT comparing the use of magnesium vs. amiodarone among 42 ICU patients with atrial tachyarrhythmias (71% with AF or atrial flutter). Magnesium was provided as a bolus of 0.037 g/kg followed by an infusion of 0.025 g/kg/hr for 24 hours. The infusion was adjusted to target a therapeutic serum magnesium concentration of 1.4-2.0 mM (3.3-4.8 mg/dL). Patients with baseline creatinine > 3.4 mg/dL or urine output <400 ml/day were excluded, unless on continuous renal replacement therapy. For patients with moderate renal insufficiency (Cr 2.3-3.3 mg/dL and/or urine output <40 ml/hour), the infusion rate was decreased by a factor of two.
.
.
Magnesium was more effective in conversion to sinus rhythm than amiodarone (figure above). Blood pressure was stable in both groups. Among patients who did not convert to sinus rhythm, heart rate reduction was similar in both groups (an average of ~19 b/m). However, this heart rate analysis excluded three patients in the magnesium group who needed to cross over to the amiodarone group due to uncontrolled ventricular rate.
.
This study has substantial limitations, most notably inclusion of various atrial tachyarrhythmias (e.g. acute AF, chronic AF, multifocal atrial tachycardia). Nonetheless, it does support the safety and efficacy of magnesium among sick ICU patients (average Apache II score of 22). It is notable that most of the patients in the magnesium group (17/21) responded to this therapy and did not require additional antiarrhythmic agents.
.
Sleeswijik 2008
.
This is a retrospective study of 29 critically ill patients with new-onset fast AF treated with a protocol involving magnesium with additional amiodarone if needed. First, patients were treated with a magnesium infusion as previously described by Moran (0.037 g/kg over 15 minutes followed by 0.025 g/kg/hr). After one hour, an amiodarone infusion was added if patients failed to respond (defined as ongoing AF with heart rate >110 b/m).
.
Following one hour of magnesium infusion, 16/29 patients responded (seven cardioverted to normal sinus rhythm and nine remained in AF with a heart rate <110 b/m). With ongoing magnesium infusion, all of these 16 patients eventually converted to sinus rhythm. Of the remaining 13 magnesium nonresponders, 11 cardioverted in response to amiodarone. The overall 24-hour cardioversion rate was 90%. AF did recur in 24% of patients (2 magnesium responders and 5 nonresponders), but in all cases this was successfully treated and none of the patients were discharged with AF.
.
There was absolutely no difference in baseline serum magnesium levels between patients who did and didn't respond to magnesium. This illustrates that magnesium may be effective even in patients with a normal baseline magnesium level (similar to torsade de pointes).
.
This study is limited by its retrospective observational design. Nonetheless, it provides support for the efficacy and safety of magnesium in critically ill patients (average Apache II score of 19). Another limitation of this study is that the magnesium infusion was stopped prematurely in patients who failed to respond to magnesium within one hour, which may have limited the efficacy of the magnesium-amiodarone combination (3).
.
Cardiac magnesium protocol: Putting evidence into practice
.
Despite safety and efficacy, magnesium infusions are rarely used for AF. The limitation on using magnesium is primarily logistic: it can be challenging to initiate and monitor a magnesium infusion. Publications on magnesium infusions used complex weight-based doses which are difficult to replicate at the bedside. In order to facilitate the safe use of magnesium infusions, the magnesium protocol below was developed. This is based on Moran and Sleeswijick et al., but it actually utilizes lower magnesium infusion rates to improve the margin of saftey (in our experience, this lower infusion rate remains adequate to achieve the target serum magnesium level).
.
.
In renal failure (i.e., GFR<30 ml/min), there is an increased risk of magnesium accumulation. Since these patients excrete less magnesium in their urine, using an infusion may be unnecessary. Instead, it might be safer and easier to replete magnesium with incremental doses while following serum levels.
.
Even if the patient cardioverts or improves prior to completion of the 24-hour infusion, it may be advisable to continue the full infusion. Magnesium has been shown to prevent AF, so completing a total-body magnesium load might reduce the risk of recurrence.
.
Safety
.
Magnesium infusions appear very safe in patients with sufficient renal function (e.g. GFR >30 ml/min) when protocolized and monitored adequately. Similar doses of magnesium have likewise been shown to be safe in critically ill patients with subarachnoid hemorrhage. Magnesium arguably has the best safety profile of any drug used for AF. It may cause minor flushing, tingling, or fatigue. Hypotension or bradycardia are rare complications, less common than with amiodarone or calcium channel blockers (Ho 2007). Unlike most anti-arrhythmic drugs, magnesium has no pro-arrhythmic effects, but instead decreasesthe risk of torsade de pointes (Ganga 2013). Magnesium infusions cause small decreases in serum calcium, of unclear clinical significance (Muroi 2008).
.
Where does magnesium fit in the greater context of AF treatment?
.
The data on magnesium is sparse, so exactly where this may fit into a management scheme for AF remains unclear. The following are situations where magnesium may be particularly useful:
- Other therapies are contraindicated (e.g. patients in whom hypotension limits the ability to use beta-blockers or calcium-channel blockers). Aside from baseline hypermagnesemia or neuromuscular disease (e.g. myasthenia gravis), there are few contraindications to magnesium.
- Adjunctive agent for chemical cardioversion
- Patients with hypomagnesemia
- Critically ill patients (who may have a higher rate of subclinical magnesium deficiency than other populations)
- AF refractory to conventional therapies
More on this next week.
.
Parting shot: Magnesium infusion for Torsade de Pointes (TdP)
.
This same magnesium infusion protocol is useful for Torsade de Pointes. Although magnesium is currently first-line therapy for torsade, patients often receive only 2-4 grams. This dose may only have temporary effects on the magnesium level, leaving patients at risk for recurrent cardiac arrest. The concept of a magnesium infusion for torsade is over two decades old; having a protocol merely translates this concept into safe bedside clinical practice (Tzivoni 1988).
.
.
- Available evidence suggests that magnesium administration for atrial fibrillation may promote both rhythm and rate control.
- Magnesium may be the safest drug available for AF, when dosed and monitored appropriately.
- Magnesium is located predominantly within cells, so serum magnesium may be a poor measure of intracellular magnesium depletion. Rapid repletion of total body intracellular magnesium stores requires a magnesium infusion.
- Having a cardiac magnesium infusion protocol is very useful for torsade de pointes, where it may reduce the risk of recurrent cardiac arrest.
Stay tuned: Will explore a management strategy for new-onset AF in critically ill patients next week. Spoiler alert: It will involve magnesium.
More information
- EMCrit: The crashing AF patient
- Best Bets review: IV magnesium for AF
- Please note: The posts on this blog are oriented towards critically ill patients who develop AF as a complication of their critical illness. If you're looking for information about outpatients who presents to the ED with AF as their primary problem, look at other FOAM resources e.g. ERCastand EMCases.
Notes
[1] Magnesium is poorly absorbed via an oral route, where it acts mostly as a cathartic agent. Although oral magnesium may be used as maintence therapy, this is not a viable approach to rapidly administering magnesium to a critically ill patient. Technically speaking it might be possible to replete total body magnesium with frequent intramuscular magnesium injections, but that would be painful.
[2] Ho et al. included data from Walker et al. twice, counting two different time-points separately.
[3] Among patients who failed to respond to magnesium within one hour, the magnesium infusion was stopped when magnesium levels were above 2.4 mg/dL. Nearly all patients treated with a magnesium infusion will rapidly achieve this levels, so this approach may cause premature termination of the magnesium infusion within a few hours.
[4] The following image has been cropped to optimize the ability to paste into an electronic chart:
Image credits: https://en.wikipedia.org/wiki/Flare#/media/File:Flare_0.jpg
Latest posts by Josh Farkas (see all)
- Pulmcrit wee: The cutoff razor - April 15, 2024
- PulmCrit Blogitorial – Use of ECGs for management of (sub)massive PE - March 24, 2024
- PulmCrit Wee: Propofol induced eyelid opening apraxia – the struggle is real - March 20, 2024









Hi ! Thank you for this straightforward review ! On a related topic, I wrote some line about magnesium & anesthesia on my french blog. here is the link translated by google : https://goo.gl/Zuh7D7
but the greatest debate is WHEN treating AF 🙂 https://goo.gl/IDLHLO
cheers !
This comment has been removed by the author.
This comment has been removed by the author.
Thanks! See what you think about the literature. The individual papers have limitations, but on the whole they may be reasonably persuasive. Overall the evidence supporting the safety of magnesium is stronger than evidence supporting efficacy.
This isn't very scientific, but in practice I've seen some patients who respond poorly to other treatments and only finally settle down after receiving magnesium. There may be inter-individual differences in the way patients respond to magnesium. Unfortunately that would be difficult to detect in most RCTs.
As usual, my mind is blown. I'll have to take a look at the primary literature before trying this, but thank you so much for bringing this to my attention!
Fantastic article Josh. Funny enough, soon after reading this blog post, I had a patient come in (no prior cardiac history) Afib w/ HRs in the 160-180s, maxed out on a Dilt ggt (which had been on hours in the ED). I gave him a mag load and put him on an infusion, and within hours he converted to a NSR. It is always great to see theory being put to practice and working beautifully. It is a relatively benign and cheap treatment, thus very attractive. Thanks for taking the time to make these posts, as you clearly put a… Read more »