CONTENTS
- Rapid Reference 🚀
- Diagnosis
- Evaluating the cause of pancreatitis
- Risk stratification – who needs ICU?
- Treatment
- Hypertriglyceridemic pancreatitis
- Podcast
- Questions & discussion
- Pitfalls
evaluation to guide etiology & management 📖
- Labs:
- Electrolytes including calcium.
- Complete blood count with differential.
- Triglyceride level.
- Liver function tests.
- Imaging: RUQ ultrasound.
- Review of medication list for potentially causative drugs.📖
ERCP 📖
- Not routinely indicated.
- May be considered if evidence of ascending cholangitis or choledolithiasis (e.g., markedly elevated bilirubin, dilation of the common bile duct).
resuscitation 📖
- Use the same resuscitative strategy as for septic shock (e.g., moderate fluid, early vasopressors if needed).
- Avoid large-volume resuscitation (e.g., fluid balance >3-4 liters positive).
analgesia 📖
- Start with:
- Opioids may worsen ileus, limit them as able.
nutrition 📖
- Non-hypertriglyceridemic:
- Non-intubated: Low-fat diet, as tolerated.
- Intubated: Start enteral nutrition as soon as hemodynamically stabilized.
- Hypertriglyceridemic pancreatitis: Strictly zero-fat diet.
hypertriglyceridemic pancreatitis: additional therapies
- Discontinue medications that may cause hypertriglyceridemia. 📖
- Gemfibrozil 600 mg BID.
- Gentle dextrose/insulin infusion. 📖
- Pain:
- Typically in epigastric area or left upper-quadrant, may radiate to the back, may be relieved by sitting.
- Epigastric tenderness on exam is usually present.
- Persistent nausea/vomiting.
- Hemorrhagic pancreatitis may cause Cullen Sign and Grey Turner Signs (figure below). If seen, this suggests a high disease severity.(Vincent 2023)
lipase
- Sensitivity and specificity are ~90% for acute pancreatitis.
- Causes of elevated lipase include:
- Pancreatic disease of any sort (e.g., pancreatitis, pseudocyst, cancer).
- Intestinal obstruction/pseudo-obstruction, perforation, duodenal ulceration, ischemia.
- Biliary disease (cholecystitis, cholangitis, choledocholithiasis).
- Renal failure.
- Heparin therapy (through activation of lipoprotein lipase).(33556276)
- Elevations of lipase due to diseases other than pancreatitis tend to be less than three times the upper-limit normal.
- Very high lipase values are more specific for a diagnosis of pancreatitis.
- Higher lipase values don't correlate with worse prognostic outcome. So severely elevated lipase values may seem scary, but they shouldn't actually be.
amylase
- Lipase has replaced amylase for the diagnosis of pancreatitis.
- There is no point in checking an amylase level.
early CT if necessary to clarify the diagnosis:
- CT is sensitive and specific for pancreatitis (>90%), also providing information about severity.
- If the patient definitely has pancreatitis (based on typical history, exam, and labs), then there is no reason to get an early CT scan (it won't affect management).
- For patients in shock, CT scan is often sensible to exclude a focus of intra-abdominal sepsis.
late CT scan for complications:
- The primary role of CT scan in pancreatitis is to look for complications if the patient deteriorates later in their course (after several days). For example, CT scan may help evaluate for infected necrosis and pseudocyst.
diagnostic criteria for acute pancreatitis
- At least two of the following are required:
- (1) Elevation of lipase >3 times upper limit normal (i.e., >~750 U/L).
- (2) Clinical history and examination suggestive of pancreatitis (e.g., epigastric abdominal pain, nausea/vomiting).
- (3) Imaging evidence of pancreatitis on CT, MRI, or ultrasound.
- ⚠️ Patients not meeting these criteria don't have pancreatitis and should not be treated for it!
diagnostic criteria for hypertriglyceridemic pancreatitis
- (1) Acute pancreatitis (as defined by criteria above).
- (2) Triglyceride level is >1,000 mg/dL (>11.2 mM):
- This cutoff is somewhat arbitrary. For example, one study involving 95 patients with hypertriglyceridemic pancreatitis suggested using a cutoff of >1,772 mg/dL (>20 mM).(21906399)
- The higher the triglyceride level is, the more likely it is to be causing pancreatitis.
- Timing of measuring the triglyceride level is also relevant, since levels will drop rapidly over time.
- (3) There is no other obvious cause of pancreatitis
- This includes the absence of gallstone obstruction or medications which are likely to cause pancreatitis
- ⚠️ Diagnosing hypertriglyceridemic pancreatitis isn't quite as simple as merely measuring a triglyceride level. For example, there are many people in the community with hereditary hypertriglyceridemia who are happily living their lives with triglyceride levels >1,000 mg/dL. If such a person were to develop a gallstone obstructing their pancreatic duct, they would have pancreatitis and hypertriglyceridemia – but the hypertriglyceridemia wouldn't really be the cause of their pancreatitis.
common causes of acute pancreatitis
- Gallstones (~40-50%).(30243452)
- Alcoholism (~30%).
- Hypertriglyceridemia (~10%).
- Hypercalcemia.
- Medications include:(29736167)
- Antibiotics: tetracyclines, sulfonamides, pentamidine, HIV medications, isoniazid, metronidazole.
- Immunosuppressives: azathioprine, sulfasalazine, mesalamine, 6-mercaptopurine.
- Cardiac: amiodarone, losartan, furosemide, pravastatin, simvastatin.
- Valproic acid.
- All-trans-retionic acid (ATRA).
- Glucagon-like peptide-1 agonist therapy for diabetes.
- Posterior penetrating ulcer.
- Trauma.
- Iatrogenic:
- ERCP.
- Endoscopic ultrasound (EUS) with fine needle aspiration.
- Surgery (including aortic surgery or CABG).
- Radiation therapy,
- Pancreatic malignancy:
- Intraductal papillary mucinous neoplasms (IPMNs).
- Adenocarcinoma.
- Cystic fibrosis.
labs to evaluate the cause of pancreatitis:
- Calcium: Rarely, hypercalcemia is a rare cause of pancreatitis. More commonly, pancreatitis may cause hypocalcemia (which can occasionally be symptomatic).
- Triglyceride level: >1,000 mg/dL (>11.2 mM) suggests hypertriglyceridemic pancreatitis.
- Liver function tests: Significantly elevated bilirubin and alkaline phosphatase suggest obstruction, raising a possible concern of simultaneous ascending cholangitis.

right upper quadrant ultrasonography
- This should be obtained on all pancreatitis patients.(10580962)
- Gallstones may suggest gallstone pancreatitis.
- The most important finding is size of the common bile duct.
Most pancreatitis patients have mild disease and can be admitted to the ward, but some require ICU admission. This is tricky because pancreatitis patients may look OK initially, but deteriorate later.
edematous vs. necrotizing pancreatitis
- Pancreatitis can be divided into two categories:
- Interstitial edematous pancreatitis (90%) – diffuse inflammation of the pancreas, tissue remains viable.
- Necrotizing pancreatitis (10%) – areas of pancreatic tissue become necrotic.
- The diagnosis of necrotizing pancreatitis is generally made based on contrast CT scan, which shows a lack of blood flow to necrotic areas. (Note, however, that CT scan shouldn't be obtained solely for this purpose.)
- Necrotizing pancreatitis is more worrisome, as these patients are at risk for developing multiorgan failure or superinfection of the devitalized pancreatic tissue (infected pancreatic necrosis). The mortality rate of necrotizing pancreatitis is 17%, much higher than the mortality of interstitial edematous pancreatitis at 3%.(29736167)
scoring systems
- Numerous scoring systems are available to predict outcomes. However, it's unclear whether scoring systems add substantively to clinical judgement.
- APACHE-II score might be the best scoring system, with scores >7 predicting severe acute pancreatitis [organ failure(s) persisting >48 hours]. Unfortunately, its performance is far from perfect (with sensitivity of 65% and specificity of 76%).🧮
- The Ranson score can't be calculated until after 48 hours, so it plays no role in up-front risk stratification.
neutrophil/lymphocyte ratio (NLR)
![]()
- NLR is a global indicator of physiological stress which is easily calculated from an admission blood count.🌊 Higher NLR values predict severe pancreatitis (area under ROC curve of ~0.75) and mortality (area under ROC curve ~0.8).(27631223, 28638228, 28878366, 29030078, 28348184) NLR seems more accurate than C-Reactive Protein (CRP), a lab test which is occasionally recommended for prognostication in pancreatitis.(28348184, 29370777)
- The table below provides a rough guide to interpreting NLR in this context.(27631223) Prognostication shouldn't be based solely on NLR, but this may provide another bit of information that contributes to the global assessment.
 possible indications for ICU admission?
possible indications for ICU admission?
- Organ failure(s), including:(33464002)
- Acute kidney injury, including substantially reduced urine output.
- Marked delirium.
- Hypotension, bradycardia, or tachycardia.
- Significant tachypnea or increased work of breathing.
- Hypoxemic respiratory failure.
- Hypertriglyceridemic pancreatitis (triglyceride >1,000 mg/dL or >11.2 mM) should be considered for insulin infusion in the ICU. 📖
Historically, pancreatitis has been treated as a unique disease. It was feared by intensivists, who skittishly deferred management to gastroenterologists and surgeons. Pancreatitis was treated with vast quantities of fluid, bowel rest, nasogastric tube drainage, parenteral nutrition, and prophylactic antibiotics – a wholly bizzare potpourri of therapies inconsistent with basic principles of critical care. Only recently have we begun the hard work of dispelling these harmful treatments.
principle of pancreatoseptic equivalence
- Severe pancreatitis and septic shock are extremely similar processes (both vasodilatory shock states involving profound systemic inflammation and endothelial dysfunction).
- The treatment for severe pancreatitis should follow the same principles as the treatment of septic shock.
- Evidence gaps regarding how to manage severe pancreatitis can be filled with experience gained from the treatment of septic shock.
This is probably not entirely accurate, but it's a huge advance compared to the bizarre ways in which pancreatitis has been treated over the past few decades.
- Hypertriglyceridemic pancreatitis: Discussed further here.📖
- Medication-induced: Stop potentially offending medications.
- Hypercalcemia: Treat this aggressively (e.g., with bisphosphonate & calcitonin).📖
- Gallstone-induced: Ideally, delayed cholecystectomy later on during the patient's admission for acute pancreatitis.
- The American Gastroenterological Association recommends against routine urgent ERCP in patients with acute biliary pancreatitis without cholangitis. Most studies of ERCP have failed to show benefit.
- The strongest indication for ERCP is definite or suspected ascending cholangitis (which sometimes occurs simultaneously with pancreatitis, serving as a focus of septic shock). Evidence of cholangitis may include:
- Dilation of the common bile duct.
- Significantly elevated & rising bilirubin.
- ERCP may also be considered for patients with evidence of persistent choledocholithiasis (e.g., persistently elevated bilirubin, impacted stone visualized radiographically).
- When in doubt about the need for ERCP, possible approaches are:
- (1) Follow the patient clinically, with serial monitoring of liver function tests and overall picture.
- (2) Magnetic Resonance Cholangiopancreatography (MRCP) – a noninvasive imaging modality to better define the anatomy of the biliary tract.
- (3) Endoscopic ultrasonography (EUS) may be utilized as a bedside procedure to evaluate for choledocholithiasis with high sensitivity.
traditional dogma: large volume fluid resuscitation
- Traditionally patients have been treated with massive fluid resuscitation (e.g., 250-500 ml/hr, resulting in ~8-14 liters fluid administration over the first day). This is insanity.
- There is no evidence to support massive volume administration. Available prospective studies show that more aggressive fluid administration increases rates of infection, abdominal compartment syndrome, ARDS, and death. 🌊 (19187641, 20819621)
reasonable approach?
- Nobody knows the best approach. There is little high-quality prospective data to guide this.
- A reasonable approach to resuscitation is probably similar to a septic shock resuscitation:
- Give fluid based on hemodynamic assessment (e.g., with ultrasonography). Patients may benefit from a moderate amount of fluid initially (e.g., ~2 liters total over the first day).
- Don't give much fluid after the initial resuscitation (e.g., beyond 12-24 hours). Following stabilization, it's generally wise to target an even fluid balance.
- Use vasopressors (e.g., norepinephrine) early, as needed to maintain an adequate MAP. This may reduce the amount of fluid given, thereby reducing the risk of abdominal compartment syndrome.
- Be careful about the use of fluid-responsiveness in these patients. Pancreatitis patients are usually fluid-responsive, but administered fluid may rapidly leak out of the vascular space.
- Patients will often develop renal failure due to acute tubular necrosis. This doesn't respond to additional fluid administration.
lactated ringers (LR) is preferred
- Lactated Ringers might be the fluid of choice, given one RCT in pancreatitis that found reduced inflammation when using LR compared to normal saline.(21645639)
- LR is a terrific resuscitative fluid, so it's a good choice regardless.🌊
- Opioids:
- Most patients will need some amount of PRN opioid, but this should be kept to a minimum.
- Opioids are especially problematic in pancreatitis because they promote ileus. This may become a major problem by interfering with nutrition and promoting compartment syndrome.
- Opioid infusions in particular should be avoided.
- Non-opioid analgesia is essential:
- Non-steroidal anti-inflammatory agents should be avoided, given the tendency of pancreatitis patients to develop acute tubular necrosis.
- Epidural analgesia may be an excellent option if available.
- More on multimodal analgesia in critical care here: 📖
the concept of “pancreatic rest” is dangerously misleading dogma
- Traditionally there was a concept that nutrition would stimulate pancreatic secretions and thereby worsen pancreatitis.
- Not only is this wrong, it's probably backwards.(29409760) Early enteral nutritional support (ideally within 24 hours) may improve outcomes, for example:
- Improved intestinal function (reduced rate of ileus, decreased bacterial translocation into the bloodstream).
- Reduced risk of infected pancreatic necrosis.
- Reduced hospital length of stay.
- Decreased gastrointestinal symptoms.(30243452)
nutritional support for non-hypertriglyceridemic pancreatitis
nutritional support for the non-intubated patient
- Oral diet may be started immediately (as clinically tolerated).
- It's fine to start with a low-fat diet (rather than a clear-liquid diet).
- Some patients are unable to tolerate food (e.g., due to pain or emesis). This may be observed for a couple days. If the patient still isn't eating after 3-5 days, a small-bore nasal feeding tube may be placed to provide nutrition.(25409371) It's ideal to place this in a post-pyloric location, but gastric feeding is also fine.
nutritional support for the intubated patient
- Enteral tube feeding should be started immediately after the initial resuscitation. Start feeds at a low level (10-20 ml/hr) and advance as tolerated.
- It is controversial whether to feed the stomach (e.g. via nasogastric/orogastric tube) or to use a post-pyloric tube.
- RCTs show no differences in outcome, so either route is fine.
- Among intubated patients it's usually easier to place an orogastric tube, so this route is often used initially.
- If the patient has problems with gastroparesis or vomiting, then switching to a post-pyloric tube may be helpful.
- 💡Intubated patients with pancreatitis can generally be fed in the same fashion as other critically ill patients.📖 The only exception to this is hypertriglyceridemic pancreatitis, who should receive fat-free enteral nutrition.📖
- Pancreatitis causes exocrine pancreatic dysfunction in a majority of patients initially, leading to malabsorption.(33464002) This may be managed using pancreatic enzyme supplements or semi-elemental tube feeding formulations.
total parenteral nutrition should be avoided
- RCTs in pancreatitis have shown harm from parenteral nutrition. This has been shown to increase the risk of infected pancreatic necrosis and multi-organ failure.(29409760)
- Parenteral nutrition should be used only as a last resort, when enteral nutrition is impossible.📖
nutritional support for hypertriglyceridemic pancreatitis
fat-free enteral nutrition is probably ideal for hypertriglyceridemic pancreatitis
- Oral diet may be started immediately if tolerated clinically (e.g., in the absence of nausea and vomiting).
- Cessation of oral fat intake may be important to help triglyceride levels fall. Thus, a truly non-fat diet might be beneficial initially.
- For nonintubated patients, this could include foods such as fruit and pasta that are extremely low in fat.
- For intubated patients, modular protein supplementation could be provided in addition to intravenous dextrose.
- Once triglyceride levels have fallen (typically after ~3-4 days), patients may be advanced to a low-fat diet.
avoid antibiotics in the first week
- There are many parallels between sepsis and pancreatitis. These will cause the pancreatitis patient to look infected upon arrival (e.g. pancreatitis commonly causes fever, leukocytosis, hypotension, and vasodilatory shock). However, this is generally a reflection of sterile inflammation rather than true infection.
- Historically there was a concept that prophylactic antibiotics could prevent the development of infected pancreatic necrosis. This has been debunked and should not be used. Up-front antibiotics will select out resistant organisms, which cause problems later on (when true infection actually does occur).
- Antibiotics should generally be avoided during the first week, with the following exceptions:
- (1) The diagnosis of pancreatitis is unclear and there is concern for septic shock with a focus of infection elsewhere.
- (2) The patient has coexisting ascending cholangitis (which is a true bacterial infection and requires decompression & antibiotics).
- Infectious complications of pancreatitis (e.g., infected necrosis) are rare during the first week. During this time frame, inflammatory symptoms (e.g., fever, leukocytosis) likely reflect sterile pancreatic inflammation.
Necrotizing pancreatitis can cause acute necrotic collections, infected pancreatic necrosis, and walled-off necrosis. Alternatively, interstitial edematous pancreatitis may cause acute peripancreatic fluid collections and pseudocysts.
acute peripancreatic fluid collection (APFC)
- Defined as a homogeneous fluid collection occurring adjacent to the pancreas within four weeks of interstitial edematous pancreatitis (i.e., not necrotizing pancreatitis).🌊
- Unlike a pseudocyst, there is no wall encapsulating the fluid.
- Superinfection can occur, but this is rare.
- Most collections resolve spontaneously without intervention. If the collection persists, it may mature into a pseudocyst (see below).
pancreatic pseudocyst
- Defined as encapsulated fluid collections with an inflammatory wall, usually occurring >1 month after interstitial edematous pancreatitis. On CT imaging, they should appear homogeneous, without any solid component.
- Pseudocysts will usually resolve spontaneously. If asymptomatic, pseudocysts may be observed with serial imaging.
- Complications resulting from pseudocysts may include: (31791953)
- Gastric or duodenal obstruction (most common).
- Biliary obstruction.
- Infection of the cyst.
- Rupture leading to pancreatic ascites.
- Bleeding, including erosion of surrounding vessels (e.g., splenic or gastroduodenal arteries).
- Symptomatic pseudocysts may be drained endoscopically, surgically, or percutaneously. Endoscopic drainage is generally preferred if technically possible. However, percutaneous drainage may be preferred for fragile patients who cannot tolerate any other procedure – despite an increased risk of fistula formation.(31192242)
acute necrotic collection (ANC)
- Defined as fluid collections adjacent to necrotizing pancreatitis that occur within the first four weeks. Unlike acute peripancreatic fluid collections:
- These may contain some heterogeneous, non-liquified material.
- Collections can be intrapancreatic and/or extrapancreatic.
- The risk of infection may be higher than with acute peripancreatic fluid collection (see section below on infected pancreatic necrosis).
- If a sterile necrotic collection persists, it may mature into walled-off necrosis (WON) after about a month.
- Most collections are sterile and will resolve with conservative management.(31192242) Acute necrotic collections may develop into infected pancreatic necrosis or walled-off sterile necrosis (with management described below).
infected pancreatic necrosis
- This peaks about 10-14 days after the onset of pancreatitis. The classic presentation would be a patient who initially improves, but subsequently deteriorates with worsening sepsis.
- Investigation typically begins with repeat CT scan. Occasionally, radiologic features may be diagnostic (e.g., gas within pancreatic tissue implies infection).
- Fine-needle aspiration to determine whether infection is present is routinely used at some centers and recommended in the Canadian guidelines for acute pancreatitis.(27007094) However, empiric antibiotics are favored at other centers due to fear of introducing infection into the pancreas during fine-needle aspiration.(28857624)
- Traditionally a carbapenem (e.g., meropenem) as used for improved penetration of the pancreas. However, other antibiotics also penetrate the pancreas well (e.g., cefepime/metronidazole and probably piperacillin-tazobactam).(18333238, 18809943) Given that these patients often remain in the ICU for some weeks, using piperacillin-tazobactam initially (instead of a carbapenem) could delay the selection of resistant pathogens.
- A team approach is required, including pancreatic surgeons, interventional radiologists, and invasive gastroenterologists. Ideally this should be managed at a large center which offers a range of minimally invasive debridement techniques.(29736167)
- The PANTER trial supported a strategy of beginning with percutaneous drainage, and stepping up to more invasive therapy if needed. Notably, solely percutaneous drainage was required in a third of patients.(20410514) However, endoscopic strategies may result in a lower risk of pancreatic fistula formation.(31791953)
walled-off necrosis (WON)
- Defined as an encapsulated collection of pancreatic and/or peripancreatic necrosis with a well-defined inflammatory wall. Maturation usually requires >4 weeks.
- Aside from the presence of a wall, this has similar features compared to an acute necrotic collection:
- May contain heterogeneous contents, including liquid and solid components.
- May be intrapancreatic and/or extrapancreatic.
- Sterile necrosis can resolve without intervention. Alternatively, management may include percutaneous drainage, endoscopic drainage, or surgical drainage. Minimally invasive techniques are increasingly being utilized.(31791953)
procalcitonin in pancreatitis
- Although procalcitonin is often conceptualized as a test for bacterial sepsis, it can be elevated in pancreatitis as well (as might be expected based on similarities between these two conditions). Procalcitonin may potentially be used for two purposes:
- (1) Risk stratification
- Greater procalcitonin elevation reflects more severe inflammation, which may predict a more severe disease course.
- Elevation of procalcitonin >0.5 ng/mL predicts severe pancreatitis with moderate reliability (sensitivity 73%, specificity 87%).(19541012)
- (2) Diagnosis of infected pancreatic necrosis
- Pancreatitis alone generally doesn't cause profound elevation in procalcitonin. Therefore, a markedly elevated procalcitonin level (e.g. >3.5 ng/ml) is suggestive of infected pancreatic necrosis.(17457167, 18470712)
- Other causes of procalcitonin elevation include renal failure and other foci of nosocomial infection (e.g., line infection, pneumonia).
- The value of procalcitonin for infected pancreatic necrosis is likely as a rule-out test (e.g., a low procalcitonin argues against infected necrosis, whereas an elevated value is nonspecific). This might be useful in avoiding unnecessary antibiotic courses or invasive procedures in patients at low risk for true infection. Further prospective evidence is needed to validate this.
- Compartment syndrome can cause deterioration and multi-organ failure.📖
- This is largely an iatrogenic complication, due to the use of excessive volumes of crystalloid. As we are moving away from large-volume resuscitation of pancreatitis, this seems to be less of a problem.
- Hemorrhage may result from erosion of arteries near the pancreas (especially the splenic or gastroduodenal arteries).
- Front-line investigation is CT angiography, which may be able to identify the bleeding vessel. For patients with active hemorrhage, angiographic embolization is the preferred treatment.(31791953)
Hypertriglyceridemic pancreatitis occupies an uncomfortable position in the medical literature, which is typical for many critical illnesses. Hypertriglyceridemic pancreatitis is uncommon but not rare, accounting for perhaps ~8% of patients with acute pancreatitis. In the United States, with trends towards increasing obesity, hypertriglyceridemic pancreatitis is likely to become more common over time. Thus, any sizable ICU will encounter this disorder regularly and must have expertise in dealing with it.
However, hypertriglyceridemic pancreatitis is uncommon enough that almost no high-quality evidence exists regarding it. Case reports and recommendations in the literature abound, yet contradict one another. Practice varies widely from being extremely aggressive (e.g., immediate plasmapheresis) to being very conservative (e.g., using only subcutaneous insulin). Most patients will improve with only supportive care, so it's easy for different authors to obtain case series of patients who appear to respond to their preferred therapy. To date, only a single prospective RCT has been performed to rigorously test any of these therapies (and – spoiler alert – the more conservative treatment arm had superior outcomes).(27574886)
The treatment strategy described below attempts to cut a middle ground through this literature, with a strategy which is reasonably aggressive yet fairly noninvasive. In the absence of definitive evidence, this is only one of many reasonable therapeutic approaches.
overview
diagnosis of hypertriglyceridemic pancreatitis
- Triglyceride levels should be checked for any patient with pancreatitis.
- Criteria for hypertriglyceridemic pancreatitis are listed above. 📖
management
- Overall, the management should be nearly the same as the management of other patients with acute pancreatitis. However, there are a few salient differences:
- (1) Discontinue/avoid medications that may cause hypertriglyceridemia (more on this below).
- (2) Gemfibrozil 600 mg BID should be started once patients can take oral medication (or another similar fibrate).
- (3) Gentle dextrose/insulin infusion may be considered.
- (4) Nutritional support should avoid fat administration (more on this above: 📖).
- ⚠️ Avoid doing crazy things, such as:
- Lipopheresis to remove fat.
- Overly aggressive insulin infusions.
common causes of hypertriglyceridemia
- Diabetes (predominantly type II), obesity, and metabolic syndrome.
- Alcoholism (it is often unclear whether pancreatitis is due to alcoholism, hypertriglyceridemia, or both).
- Hereditary hyperlipoproteinemia (usually types IV and V).
- Pregnancy.
- Hypothyroidism.
- Medications (listed in decreasing order of evidentiary strength)(30531242)
- I (demonstrated to cause hypertriglyceridemia with positive rechallenge):
- Clomiphene.
- Estrogen and related products.
- In vitro fertilization.
- Nadolol.
- Tamoxifen.
- Furosemide.
- Propofol.
- II (at least two cases in literature, with consistent latency):
- Isotretinoin.
- Everolimus.
- Olanzapine.
- Quetiapine.
- Ritonavir.
- III (at least two cases in the literature, with no consistent latency):
- All-trans retinoic acid.
- IV (lower level evidence):
- Asparaginase, pegaspargase.
- Atenolol.
- Capecitabine.
- Entecavir.
- Estramustine phosphate.
- Lisinopril.
- Mirtazapine.
- Montelukast.
- Prednisone.
- Tocilizumab.
- Venlafaxine.
- I (demonstrated to cause hypertriglyceridemia with positive rechallenge):
dextrose infusion (+/- insulin)
general concept of dextrose infusion
- The goal of the dextrose infusion is to establish an anabolic state (i.e., a state where metabolic substrates are being stored in the form of glycogen and fat, rather than being broken down). Establishing an anabolic state will shut off the production of free fatty acids in adipose tissue.
- ⚠️ The goal is not necessarily to reduce the triglyceride level (since the best available evidence suggests that dextrose/insulin is ineffective at reducing triglyceride levels).
- Clinically, we can tell that the patient is in an anabolic state if we are giving them dextrose and they aren't becoming hyperglycemic. This implies that the appropriate dose of insulin may be whatever dose is required to prevent hyperglycemia in the face of a significant dextrose infusion.
- The amount of insulin required will vary widely depending on how insulin-resistant any individual patient is and whether they are capable of synthesizing insulin. Patients with greater insulin resistance (e.g., type II diabetes) will require more insulin. Patients with a normally functioning pancreas may require no insulin.
- Below is a simple protocol for achieving this. There is no prospective evidence supporting this protocol.
one protocol for insulin infusion in hypertriglyceridemic pancreatitis
- Before starting:
- Replete potassium (targeting ~5-5.3 mEq/L).
- Replete phosphate (if hypophosphatemia is present).
- Initiation:
- Start peripheral D10W at ~100-125 ml/hour. Continue D10W infusion at a fixed rate. (If the patient has central access, then D20W or D50W may be used at a lower volume, but establishing central access solely for this purpose shouldn't generally be needed.)
- Start an insulin infusion if necessary necessary to achieve a serum glucose of ~140-220 mg/dL. Insulin requirements vary widely (e.g., some patients may not require any exogenous insulin, because they are synthesizing their own insulin.)
- Maintenance
- (1) Follow electrolytes (including magnesium and phosphate) q6hr. Replete aggressively.
- (2) Follow glucose q1hr.
- (3) Follow volume status. Administer a loop diuretic (e.g., IV furosemide or bumetanide) as necessary to avoid volume overload.
- (4) Follow the triglyceride level daily.
- When to stop the insulin infusion
- This is an evidence-free zone.
- If the triglyceride level falls below 1,000 mg/dL (<11.2 mM), then the insulin infusion can likely be stopped.
- If the patient is making solid clinical improvement, the insulin infusion may probably be stopped after 24-48 hours (regardless of the triglyceride level).
- How to stop the insulin infusion
- If the patient requires chronic insulin for diabetes, then their basal chronic insulin should be initiated. Some patients with newly diagnosed Type-II diabetes will require initiation of long-acting insulin (sometimes at high doses).
- The insulin and dextrose infusions may be simultaneously weaned off, with careful attention to the glucose level.
background: physiology of hypertriglyceridemic pancreatitis
 biochemistry
biochemistry
- Triglycerides consist of three fatty acids fused to a glycerol backbone (above figure, left). Triglycerides are a form of fat which is trafficked around the body stuffed together within small particles (e.g., chylomicrons or Very Low Density Lipoproteins, VLDL). Triglycerides are also the main form of fat stored within adipose tissue.
- Triglycerides within chylomicrons or VLDL particles seem to be relatively inert.
- Triglycerides may be metabolized into glycerol plus free fatty acids.
- Free fatty acids are more water-soluble, so they can dissolve in the blood directly.
- Free fatty acids appear to be more toxic than triglycerides (more on this below).
accumulation of triglycerides and free fatty acids in patients with insulin resistance

- Insulin resistance (e.g., type II diabetes) can increase levels of free fatty acids and circulating triglycerides, as shown above.(30723557)
- (1) Insulin normally promotes the storage of triglycerides in adipose tissue. Inadequate insulin activity leads to lipolysis of triglycerides in adipose cells, releasing free fatty acids into circulation. Some of these free fatty acids are converted by the liver back into triglycerides which circulate in the bloodstream in the form of Very Low Density Lipoproteins (VLDL).
- (2) Insulin normally promotes absorption of triglycerides into the fat tissue (via stimulation of lipoprotein lipase, which helps disassemble triglycerides and traffic the fatty acids into adipose tissue). Inadequate insulin activity leads to an accumulation of triglycerides in circulation (e.g., chylomicrons formed from dietary fat absorption and Very Low Density Lipoproteins formed in the liver).
- When insulin's effects are severely deficient, this physiology may also lead to ketoacid production (as free fatty acids are metabolized into ketoacids). This explains the following clinical phenomena:
- Patients presenting with diabetic ketoacidosis may have elevated levels of triglycerides. In some cases, insulin deficiency may simultaneously trigger diabetic ketoacidosis and hypertriglyceridemic pancreatitis.
how does dysregulated lipid metabolism cause pancreatitis?
- The exact mechanism causing pancreatitis isn't entirely clear. Two possible explanations are as follows:
- (1) Toxicity due to elevated triglyceride levels ?? – Very high levels of triglycerides may theoretically occlude capillaries.
- However, many patients live for years with extremely high triglyceride levels and do not develop pancreatitis, so hypertriglyceridemia alone doesn't seem to be particularly toxic.
- It's debatable whether triglycerides are toxic at all, with some authors stating that they lack inherent toxicity.(32571534)
- (2) Toxicity is due to free fatty acids – this seems more likely to be the primary cause of pancreatitis. Free fatty acids can stimulate inflammation and form conglomerates that act as a detergent to damage cell membranes.
“hypertriglyceridemic pancreatitis” is probably a misnomer
- “Hypertriglyceridemic pancreatitis” is potentially misleading, because this implies that the triglycerides cause the pancreatitis. As discussed above, this doesn't appear to be true.
- Rather than hypertriglyceridemia, it's probably the elevation of free fatty acid levels which causes pancreatitis. Thus, a more accurate term might be “free fatty acid pancreatitis.”
- Unfortunately, it's impossible to measure serum free fatty acid levels in clinical practice, whereas it is easy to measure triglyceride levels. Thus, we remain fixated on the triglyceride level – although this level probably serves as a surrogate measurement of free fatty acid levels (which may be the true culprit).
- Hypocalcemia might be an indirect measurement of elevated fatty acid levels, because the fatty acids can bind to calcium.
natural history of triglyceride levels in pancreatitis
Without any specific therapy, triglyceride levels tend to fall over time (likely related to fluid resuscitation and reduced oral fat intake). This natural fall has been well documented over decades; for example, in this study from 1991:(1787337)

Insulin infusion would be expected to accelerate the fall in triglyceride levels (e.g., due to stimulation of lipoprotein lipase, which encourages triglyceride absorption by adipose tissue). However, a recent retrospective study compared the rate of triglyceride clearance among patients treated with insulin versus patients who didn't receive insulin. No difference was detected, as shown below.(31993551) Once again, a natural drop in triglyceride levels is observed over time, precisely in line with Dominguez-Munoz et al.'s study from 1991 above.
 Why didn't insulin accelerate triglyceride clearance to any measurable extent? It seems that the primary driver of the drop in triglyceride levels over time may be reduced oral fat intake. Insulin administration may not add much to these treatments. This drop in triglyceride levels with conservative therapy only has been replicated by another recent study as well.(31077464)
Why didn't insulin accelerate triglyceride clearance to any measurable extent? It seems that the primary driver of the drop in triglyceride levels over time may be reduced oral fat intake. Insulin administration may not add much to these treatments. This drop in triglyceride levels with conservative therapy only has been replicated by another recent study as well.(31077464)
It's extremely important to realize that triglyceride levels will fall on their own, without any fancy intervention. Many case reports have demonstrated that triglyceride levels fall with various interventions (e.g., plasmapheresis). However, these reductions in triglyceride levels may simply reflect the natural history of the disease.
clinical implications of the basic science
The above model of hypertriglyceridemic pancreatitis hasn't been rigorously proven, but seems consistent with observed evidence. This model has implications for the treatment of hypertriglyceridemic pancreatitis – specifically, that treatments should focus largely on reducing the free fatty acid levels (not necessarily reducing the triglyceride level). When viewed through this lens, treatment implications include the following:
insulin therapy
- A biochemical understanding of fatty acid metabolism suggests that insulin should be the front-line therapy for hypertriglyceridemic pancreatitis. Insulin administration is the fastest way to shut off fatty acid production and reduce free fatty acid levels. Analogous to diabetic ketoacidosis, insulin administration has the ability to drop free fatty acid levels within hours.(21593106)
- Historically, insulin infusions have often been continued until the patient reached a certain triglyceride level (often <1,000 mg/dL or <11.2 mM). However, this doesn't make sense, given that insulin doesn't appear to substantially affect triglyceride levels (see above). A more sensible approach may be to simply continue an insulin infusion until the patient is making a sustained clinical recovery.
lack of utility of plasmapheresis
- Plasma exchange can be used to reduce triglyceride levels. However, the entire concept behind this may be fundamentally flawed:
- If the pathophysiological problem is predominantly free fatty acids (rather than triglycerides), then plasmapheresis is a fundamentally misguided therapy. Unlike insulin, plasmapheresis won't shut off the synthesis of free fatty acids, so plasmapheresis might actually have little effect on free fatty acid levels.
- Plasmapheresis has numerous additional drawbacks, including the following:
- It is invasive (requiring placement of a large bore hemodialysis catheter).
- Plasmapheresis is expensive and not widely available. Adopting a strategy of plasmapheresis will require greater levels of interhospital transfer (with attendant risks and costs).
- Plasmapheresis incurs delays to treatment initiation (many hours elapse between the decision to perform plasmapheresis and any reduction in the patient's triglyceride level).
- Citrate involved in plasmapheresis may decrease calcium levels (which are often already low). Alternatively, if heparin is used for anticoagulation of the dialysis circuit, this may increase the risk of hemorrhagic pancreatitis and perhaps the risk of mortality.(25047332)
- If plasma is used as replacement fluid, this carries risks of allergic reaction or disease transmission. Alternatively, if albumin is used as replacement fluid, this may exacerbate coagulopathy.
- Plasmapheresis is not supported by any clinical evidence.
- Available clinical series show that clinical outcomes from plasmapheresis are equivalent to treatment with insulin infusion or simply conservative management.(15259080, 25047332, 28233051)
- There is no clear evidence that plasmapheresis reduces triglyceride levels any faster than simply eliminating oral fat intake and providing fluid resuscitation. One retrospective series suggested equivalent reductions in triglyceride levels over time, regardless of whether plasmapheresis was used! (figure below)(28233051)
- One RCT involving 66 patients with hypertriglyceridemic pancreatitis compared insulin infusion versus high-volume hemofiltration (an extracorporal technique used to remove triglycerides which is even more effective than plasmapheresis). High-volume hemofiltration was dramatically effective at reducing the triglyceride level. Hemofiltration achieved triglyceride levels <500 mg/dL within nine hours, which was much more effective than the insulin infusion. However, patients treated with insulin infusion did better clinically (with lower rates of severe pancreatitis and lower rates of persistent organ failure). This study arguably represents the highest quality evidence comparing filtration techniques to remove triglyceride versus an insulin infusion. Insulin was less expensive, less invasive, and clinically superior.(27574886)
- Overall, there is a notable lack of any compelling theoretical or clinical evidence to support plasmapheresis. In particular, it seems likely that insulin infusion is more effective (while simultaneously being less invasive and safer). Plasmapheresis for hypertriglyceridemic pancreatitis is analogous to using hemodialysis to treat diabetic ketoacidosis – hemodialysis will clear the ketones, but it's a fundamentally flawed approach.

lack of utility of heparin
- Historically there was some interest in using heparin as a therapy, given that heparin may up-regulate the activity of endothelial lipoprotein lipase. Unfortunately, heparin also causes endothelial lipoprotein lipase to diffuse into the bloodstream (rather than staying stuck on the capillary wall). Free lipoprotein lipase may actually tend to increase the level of free fatty acid (as opposed to endothelial-bound enzyme, which promotes the uptake of fatty acids intracellularly).
- Heparin could also promote bleeding, specifically retroperitoneal hemorrhage.
- Thus, heparin could make matters worse. In one large series of patients with hypertriglyceridemic pancreatitis undergoing plasmapheresis, anticoagulation with heparin was associated with a 10-fold higher mortality compared with patients treated with citrate anticoagulation (11% vs. 1%)(25047332)
Follow us on iTunes
The Podcast Episode
Want to Download the Episode?
Right Click Here and Choose Save-As
Podcast on hypertriglyceridemic pancreatitis:
To keep this page small and fast, questions & discussion about this post can be found on another page here.
Pancreatitis pitfalls:
- #1 most common error is administration of excessive volumes of fluid, causing ARDS and abdominal compartment syndrome. Unfortunately, this strategy continues to be recommended by many sources.
- Delayed initiation of nutrition, due to a desire to “rest” the pancreas.
- Fear-induced initiation of antibiotics during the first week of therapy, when superinfection is uncommon.
- Meropenem isn't required to penetrate pancreatic tissue, piperacillin-tazobactam also has good penetration.
Hypertriglyceridemic pancreatitis pitfalls:
- Failure to diagnose hypertriglyceridemic pancreatitis due to not checking triglyceride levels.
- Plasmapheresis for hypertriglyceridemic pancreatitis is expensive, invasive, and lacks any evidentiary basis. Plasmapheresis is recommended by many sources, but this is very hard to justify from an evidence-based medicine standpoint.
- Administering an insulin infusion with inadequate attention to electrolyte levels (e.g., potassium) may be dangerous. The approach to insulin in these patients should be similar to its use in diabetic ketoacidosis, with careful monitoring of electrolytes and glucose levels.
- Providing a full diet for patients with hypertriglyceridemic pancreatitis may make it impossible to reduce the triglyceride level. Thus, a truly fat-free diet is probably preferable here.
- Excessive fluid administration may lead to volume overload and intra-abdominal compartment syndrome. Follow volume status carefully for patients undergoing ongoing infusions of dextrose and insulin. Consider judicious use loop diuretics to avoid volume overload.
Guide to emoji hyperlinks 
= Link to online calculator.
= Link to Medscape monograph about a drug.
= Link to IBCC section about a drug.
= Link to IBCC section covering that topic.
= Link to FOAMed site with related information.
= Link to supplemental media.
References
- 01787337 Dominguez-Muñoz JE, Malfertheiner P, Ditschuneit HH, et al. Hyperlipidemia in acute pancreatitis. Relationship with etiology, onset, and severity of the disease. Int J Pancreatol. 1991;10(3-4):261-267 [PubMed]
- 10580962 Harvey RT, Miller WT Jr. Acute biliary disease: initial CT and follow-up US versus initial US and follow-up CT. Radiology. 1999 Dec;213(3):831-6. doi: 10.1148/radiology.213.3.r99dc17831 [PubMed]
- 15259080 Chen JH, Yeh JH, Lai HW, Liao CS. Therapeutic plasma exchange in patients with hyperlipidemic pancreatitis. World J Gastroenterol. 2004;10(15):2272-2274. doi:10.3748/wjg.v10.i15.2272 [PubMed]
- 17457167 Rau BM, Kemppainen EA, Gumbs AA, Büchler MW, Wegscheider K, Bassi C, Puolakkainen PA, Beger HG. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg. 2007 May;245(5):745-54. doi: 10.1097/01.sla.0000252443.22360.46 [PubMed]
- 18333238 Otto W, Komorzycki K, Krawczyk M. Efficacy of antibiotic penetration into pancreatic necrosis. HPB (Oxford). 2006;8(1):43-8. doi: 10.1080/13651820500467275 [PubMed]
- 18470712 Rau B, Steinbach G, Baumgart K, Gansauge F, Grünert A, Beger HG. The clinical value of procalcitonin in the prediction of infected necrosis in acute pancreatitis. Intensive Care Med. 2000 Mar;26 Suppl 2:S159-64. doi: 10.1007/BF02900730 [PubMed]
- 18809943 Bertazzoni Minelli E, Benini A, Franco L, Bassi C, Pederzoli P. Piperacillin-tazobactam penetration into human pancreatic juice. Antimicrob Agents Chemother. 2008 Nov;52(11):4149-52. doi: 10.1128/AAC.00509-08 [PubMed]
- 19187641 Mao EQ, Tang YQ, Fei J, Qin S, Wu J, Li L, Min D, Zhang SD. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl). 2009 Jan 20;122(2):169-73 [PubMed]
- 19541012 Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery. 2009 Jul;146(1):72-81. doi: 10.1016/j.surg.2009.02.013 [PubMed]
- 20819621 Mao EQ, Fei J, Peng YB, Huang J, Tang YQ, Zhang SD. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl). 2010 Jul;123(13):1639-44 [PubMed]
- 21593106 Chow CC, Periwal V, Csako G, et al. Higher acute insulin response to glucose may determine greater free fatty acid clearance in African-American women. J Clin Endocrinol Metab. 2011;96(8):2456-2463. doi:10.1210/jc.2011-0532 [PubMed]
- 21645639 Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011 Aug;9(8):710-717.e1. doi: 10.1016/j.cgh.2011.04.026 [PubMed]
- 21906399 Sandhu S, Al-Sarraf A, Taraboanta C, Frohlich J, Francis GA. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157. Published 2011 Sep 11. doi:10.1186/1476-511X-10-157 [PubMed]
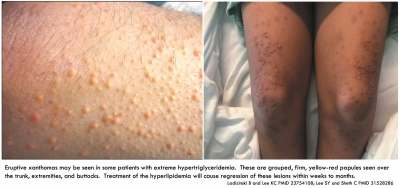
- 23754108 Ladizinski B, Lee KC. Eruptive xanthomas in a patient with severe hypertriglyceridemia and type 2 diabetes. CMAJ. 2013;185(18):1600. doi:10.1503/cmaj.130148 [PubMed]
- 25047332 Gubensek J, Buturovic-Ponikvar J, Romozi K, Ponikvar R. Factors affecting outcome in acute hypertriglyceridemic pancreatitis treated with plasma exchange: an observational cohort study. PLoS One. 2014;9(7):e102748. Published 2014 Jul 21. doi:10.1371/journal.pone.0102748 [PubMed]
- 25409371 Bakker OJ, van Brunschot S, van Santvoort HC, et al.; Dutch Pancreatitis Study Group. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med. 2014 Nov 20;371(21):1983-93. doi: 10.1056/NEJMoa1404393 [PubMed]
- 25477978 Imani F, Motavaf M, Safari S, Alavian SM. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepat Mon. 2014 Oct 11;14(10):e23539. doi: 10.5812/hepatmon.23539 [PubMed]
- 27007094 Greenberg JA, Hsu J, Bawazeer M, Marshall J, Friedrich JO, Nathens A, Coburn N, May GR, Pearsall E, McLeod RS. Clinical practice guideline: management of acute pancreatitis. Can J Surg. 2016 Apr;59(2):128-40. doi: 10.1503/cjs.015015 [PubMed]
- 27574886 He WH, Yu M, Zhu Y, et al. Emergent Triglyceride-lowering Therapy With Early High-volume Hemofiltration Against Low-Molecular-Weight Heparin Combined With Insulin in Hypertriglyceridemic Pancreatitis: A Prospective Randomized Controlled Trial. J Clin Gastroenterol. 2016;50(9):772-778. doi:10.1097/MCG.0000000000000552 [PubMed]
- 27631223 Zhang Y, Wu W, Dong L, Yang C, Fan P, Wu H. Neutrophil to lymphocyte ratio predicts persistent organ failure and in-hospital mortality in an Asian Chinese population of acute pancreatitis. Medicine (Baltimore). 2016 Sep;95(37):e4746. doi: 10.1097/MD.0000000000004746 [PubMed]
- 28233051 Miyamoto K, Horibe M, Sanui M, et al. Plasmapheresis therapy has no triglyceride-lowering effect in patients with hypertriglyceridemic pancreatitis. Intensive Care Med. 2017;43(6):949-951. doi:10.1007/s00134-017-4722-3 [PubMed]
- 28348184 Li Y, Zhao Y, Feng L, Guo R. Comparison of the prognostic values of inflammation markers in patients with acute pancreatitis: a retrospective cohort study. BMJ Open. 2017 Mar 27;7(3):e013206. doi: 10.1136/bmjopen-2016-013206 [PubMed]
- 28638228 Jeon TJ, Park JY. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J Gastroenterol. 2017 Jun 7;23(21):3883-3889. doi: 10.3748/wjg.v23.i21.3883 [PubMed]
- 28713597 Agerwala SM, Sundarapandiyan D, Weber G. Ketamine Use for Successful Resolution of Post-ERCP Acute Pancreatitis Abdominal Pain. Case Rep Anesthesiol. 2017;2017:7845358. doi: 10.1155/2017/7845358 [PubMed]
- 28857624 McSparron JI, Hayes MM, Poston JT, Seaburg LA, Morris AE, Antkowiak M, Farkas J, Athale J, Stephens RS, Dodd KW, Prekker ME, Hountras P, Cuttica MJ, Soffler M, Hibbert KA, Leclair T, Clouser R, Luks AM. ATS Core Curriculum 2017: Part III. Adult Critical Care Medicine. Ann Am Thorac Soc. 2017 Aug;14(Suppl_2):S182-S195. doi: 10.1513/AnnalsATS.201702-180CME [PubMed]
- 28878366 Han C, Zeng J, Lin R, Liu J, Qian W, Ding Z, Hou X. The utility of neutrophil to lymphocyte ratio and fluid sequestration as an early predictor of severe acute pancreatitis. Sci Rep. 2017 Sep 6;7(1):10704. doi: 10.1038/s41598-017-10516-6 [PubMed]
- 29030078 Wang Y, Fuentes HE, Attar BM, Jaiswal P, Demetria M. Evaluation of the prognostic value of neutrophil to lymphocyte ratio in patients with hypertriglyceridemia-induced acute pancreatitis. Pancreatology. 2017 Nov-Dec;17(6):893-897. doi: 10.1016/j.pan.2017.10.001 [PubMed]
- 29370777 Cho SK, Jung S, Lee KJ, Kim JW. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio can predict the severity of gallstone pancreatitis. BMC Gastroenterol. 2018 Jan 25;18(1):18. doi: 10.1186/s12876-018-0748-4 [PubMed]
- 29409760 Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018 Mar;154(4):1096-1101. doi: 10.1053/j.gastro.2018.01.032 [PubMed]
- 29736167 Garber A, Frakes C, Arora Z, Chahal P. Mechanisms and Management of Acute Pancreatitis. Gastroenterol Res Pract. 2018 Mar 15;2018:6218798. doi: 10.1155/2018/6218798 [PubMed]
- 29760856 Motov S, Drapkin J, Likourezos A, Beals T, Monfort R, Fromm C, Marshall J. Continuous Intravenous Sub-Dissociative Dose Ketamine Infusion for Managing Pain in the Emergency Department. West J Emerg Med. 2018 May;19(3):559-566. doi: 10.5811/westjem.2017.12.36174 [PubMed]
- 29870457 Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, Bhatia A, Davis FN, Hooten WM, Cohen SP. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018 Jul;43(5):456-466. doi: 10.1097/AAP.0000000000000806 [PubMed]
- 30083325 de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002 [PubMed]
- 30148167 Garg R, Rustagi T. Management of Hypertriglyceridemia Induced Acute Pancreatitis. Biomed Res Int. 2018;2018:4721357. Published 2018 Jul 26. doi:10.1155/2018/4721357 [PubMed]
- 30243452 Hammad AY, Ditillo M, Castanon L. Pancreatitis. Surg Clin North Am. 2018 Oct;98(5):895-913. doi: 10.1016/j.suc.2018.06.001 [PubMed]
- 30531242 Elkhouly MA, Salazar MJ, Simons-Linares CR. Hypertriglyceridemia-Associated Drug-Induced Acute Pancreatitis. Pancreas. 2019;48(1):22-35. doi:10.1097/MPA.0000000000001190 [PubMed]
- 30723557 Zaher FZ, Boubagura I, Rafi S, Elmghari G, Elansari N. Diabetic Ketoacidosis Revealing a Severe Hypertriglyceridemia and Acute Pancreatitis in Type 1 Diabetes Mellitus. Case Rep Endocrinol. 2019;2019:8974619. Published 2019 Jan 6. doi:10.1155/2019/8974619 [PubMed]
- 30730344 De Waele E, Malbrain MLNG, Spapen HD. How to deal with severe acute pancreatitis in the critically ill. Curr Opin Crit Care. 2019 Apr;25(2):150-156. doi: 10.1097/MCC.0000000000000596 [PubMed]
- 31077464 Berberich AJ, Ziada A, Zou GY, Hegele RA. Conservative management in hypertriglyceridemia-associated pancreatitis. J Intern Med. 2019;286(6):644-650. doi:10.1111/joim.12925 [PubMed]
- 31192242 Bezmarević M, van Dijk SM, Voermans RP, van Santvoort HC, Besselink MG. Management of (Peri)Pancreatic Collections in Acute Pancreatitis. Visc Med. 2019 Apr;35(2):91-96. doi: 10.1159/000499631 [PubMed]
- 31528286 Lee SY, Sheth CA. Eruptive xanthoma associated with severe hypertriglyceridemia and poorly controlled type 1 diabetes mellitus. J Community Hosp Intern Med Perspect. 2019;9(4):344-346. Published 2019 Sep 5. doi:10.1080/20009666.2019.1650591 [PubMed]
- 31791953 Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019 Dec 2;367:l6227. doi: 10.1136/bmj.l6227 [PubMed]
- 31993551 Dhindsa S, Sharma A, Al-Khazaali A, et al. Intravenous Insulin Versus Conservative Management in Hypertriglyceridemia-Associated Acute Pancreatitis. J Endocr Soc. 2019;4(1):bvz019. Published 2019 Nov 18. doi:10.1210/jendso/bvz019 [PubMed]
- 32422634 Gliem N, Ammer-Herrmenau C, Ellenrieder V, Neesse A. Management of Severe Acute Pancreatitis: An Update. Digestion. 2021;102(4):503-507. doi: 10.1159/000506830 [PubMed]
- 32571534 Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology. 2020;20(5):795-800. doi:10.1016/j.pan.2020.06.005 [PubMed]
- 33230385 Lee PJ, Papachristou GI. Management of Severe Acute Pancreatitis. Curr Treat Options Gastroenterol. 2020 Nov 19:1-12. doi: 10.1007/s11938-020-00322-x [PubMed]
- 33464002 Sinonquel P, Laleman W, Wilmer A. Advances in acute pancreatitis. Curr Opin Crit Care. 2021 Apr 1;27(2):193-200. doi: 10.1097/MCC.0000000000000806 [PubMed]
- 33556276 Gardner TB. Acute Pancreatitis. Ann Intern Med. 2021 Feb;174(2):ITC17-ITC32. doi: 10.7326/AITC202102160 [PubMed]
- 34562408 Vishnupriya K, Chanmugam A. Acute pancreatitis: the increasing role of medical management of a traditionally surgically managed disease. Am J Med. 2021 Sep 22:S0002-9343(21)00584-2. doi: 10.1016/j.amjmed.2021.08.021 [PubMed]