
Janus kinases (JAKs) are named after Janus, the Greek god of beginnings, gates, transitions, and duality. They're named after Janus because they contain a mirror-image structural element. However, given their role within the immune system as a multi-functional gateway for cytokine systems, the name seems apt.
The cytokine storm induced by COVID-19 has received considerable attention, regarding potential benefits of immunomodulatory therapy. To date, parenteral targeted therapies have received the most attention (especially tocilizumab which inhibits IL-6, and anakinra which inhibits IL-1). Although promising, these agents have considerable drawbacks. Mechanistically, they inhibit only a single cytokine target – a strategy which has historically failed for diseases such as sepsis, with multiple cytokines involved. As large proteins, these drugs suffer from numerous logistic constraints (high cost, limited ability to be manufactured in large quantity, need for cold storage, and requirement for IV administration).

In contrast to these somewhat awkward and focused agents, ruxolitinib offers several advantages:1–3
- Ruxolitinib blocks a shared signal transduction pathway which is used by several cytokine receptors (shown above). This could theoretically be more effective than targeting any single cytokine (amid a poly-cytokine storm).
- Oral administration.
- Ruxolitinib has a favorable safety profile, including during long-term outpatient use in fragile patients (e.g., patients with myelofibrosis or graft-versus-host disease).4,5
- As a small molecule, ruxolitinib might be more amenable to large-scale manufacture and worldwide distribution.
Cao et al. Ruxolitinib in treatment of severe COVID-19: A multi-center, single-blind, randomized controlled trial
This is a prospective, multi-center RCT performed in China.6 43 patients were randomized, with subsequent withdrawal of two patients before receiving any therapy (due to ineligibility and withdrawal of consent). Patients were randomized to receive ruxolitinib 5 mg BID or placebo.
Key inclusion criteria were:
- “Severe” COVID-19 (defined as having hypoxemia on room air or respiratory rate >30 breaths/minute)
- Not intubated at the time of recruitment
- Not pregnant or lactating
- No other active infection

41 patients were included in the final analysis. At baseline, they were well matched and moderately ill (table below; note elevated LDH and D-dimer values). The median time interval from symptom onset to randomization was quite long, at 20 days.

The use of other interventions in these patients was balanced between groups (with 70% of patients receiving steroid).

Clinical endpoints are shown below. There were no significant differences in rate of clinical improvement or mortality, although trends did favor ruxolitinib. Four patients in the control group deteriorated clinically, whereas none of the patients in the ruxolitinib group did (p=0.1). Similarly, three patients in the control group required intubation and subsequently died, whereas none in the ruxolitinib group did. Patients in the ruxolitinib group were more likely to have improvement in their 14-day follow-up CT scan.

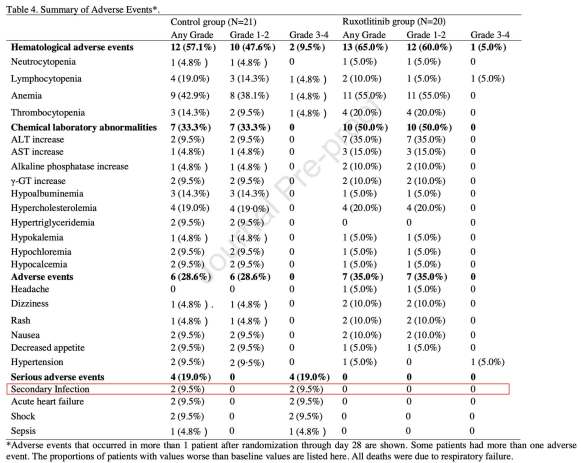
Safety endpoints showed no signals of harm. If anything, there was a trend towards more frequent infection in the control group:
Levels of 48 different cytokines were evaluated in all patients. In the ruxolitinib group all cytokine levels decreased over the first three days, whereas in the control group four cytokines increased (macrophage inflammatory protein 1-alpha, granulocyte colony stimulating factor, IFN-alpha2, and IL-1alpha). This is shown in the top left panel of the figure below (each dot represents the average change in a different cytokine level over 3 days).

Levels of several cytokines are shown above. Even in cases where the average cytokine level decreased among control patients, reductions were often greater among patients treated with ruxolitinib.
There were no differences in viral titers or viral clearance, suggesting that ruxolitinib didn’t impair the anti-viral immune response (although this analysis was limited to a total of 17 patients with measurable viral titers). Indeed, there were actually higher levels of IgM antibodies against COVID-19 among patients treated with ruxolitinib (panel F below). This raises the possibility that by suppressing an over-exuberant cellular immune response, ruxolitinib may have actually improved the humoral immune response (these responses may be in a reciprocal balance, with one suppressing the other).

Weaknesses of the study
- Two patients in the ruxolitinib group were withdrawn after randomization (one patient withdrew consent and the other patient was found to be ineligible due to having recently received chimeric antigen receptor (CAR) T-cell therapy). Post-randomization withdrawal is generally frowned upon. However, these patients didn’t actually receive ruxolitinib at all, eliminating the possibility of selective patient removal following treatment.
- 100 mg tabs of ascorbic acid were used as a placebo. Critics of vitamin C will find this amusing. The choice of vitamin C as a “placebo” isn’t well explained (perhaps these tablets had the same appearance as ruxolitinib?). During a pandemic there isn’t time to design matching placebo tablets, so we shouldn’t expect perfection here. Ultimately, 100 mg of oral vitamin C is unlikely to do much, so this is probably fine as a placebo.
- Treating physicians were not blinded to allocation, so this could have affected the selection of other treatments. However, treatments received by the two groups appeared to be essentially identical (table 2 above).
- The study was prematurely terminated when the COVID-19 epidemic in China ended (this is an inevitable limitation of many of these studies). This leads to underpowering of clinical endpoints.

- Ruxolitinib is an oral, small-molecule inhibitor of intracellular kinases (JAK1 & JAK2). It functions to block the intracellular signaling pathways involved in several pro-inflammatory cytokines.
- The most common indication for ruxolitinib is chronic therapy for myeloproliferative disorders. More recently, ruxolitinib has been used for critically ill patients with hemophagocytic lymphohistiocytosis.7–10
- Cao et al. is the first multi-center RCT evaluating ruxolitinib in COVID-19.6 Premature termination of the study leaves it underpowered to evaluate clinical endpoints. However, there are some tantalizing signals of possible benefit (e.g., non-significant mortality reduction and and barely significant improvements in CT scan resolution).
- Ruxolitinib did improve cytokine levels and C-reactive protein, indicating its ability to function rapidly as an immunomodulator.
- Immunomodulation was not found to delay viral clearance. Indeed, patients treated with ruxolitinib had higher IgM antibody levels. However, it’s possible that initiation of treatment too early in the disease course might increase viral replication (because JAK-1 is involved in interferon signaling that suppresses early viral replication).
- Further RCTs are currently underway to further evaluate ruxolitinib and the related JAK-inhibitor baricitinib.

references
- 1.Caocci G, La N. Could ruxolitinib be effective in patients with COVID-19 infection at risk of acute respiratory distress syndrome (ARDS)? Ann Hematol. Published online May 14, 2020. doi:10.1007/s00277-020-04067-6
- 2.Galimberti S, Baldini C, Baratè C, et al. The CoV-2 outbreak: how hematologists could help to fight Covid-19. Pharmacol Res. 2020;157:104866. doi:10.1016/j.phrs.2020.104866
- 3.La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. Published online June 9, 2020. doi:10.1038/s41375-020-0891-0
- 4.Sant’Antonio E, Bonifacio M, Breccia M, Rumi E. A journey through infectious risk associated with ruxolitinib. Br J Haematol. 2019;187(3):286-295. doi:10.1111/bjh.16174
- 5.Kiladjian J, Zachee P, Hino M, et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020;7(3):e226-e237. doi:10.1016/S2352-3026(19)30207-8
- 6.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. Journal of Allergy and Clinical Immunology. Published online May 2020. doi:10.1016/j.jaci.2020.05.019
- 7.Gálvez A, Javalera R. Ruxolitinib as first-line therapy in secondary hemophagocytic lymphohistiocytosis and HIV infection. Int J Hematol. Published online April 13, 2020. doi:10.1007/s12185-020-02882-1
- 8.Zandvakili I, Conboy C, Ayed A, Cathcart-Rake E, Tefferi A. Ruxolitinib as first-line treatment in secondary hemophagocytic lymphohistiocytosis: A second experience. Am J Hematol. 2018;93(5):E123-E125. doi:10.1002/ajh.25063
- 9.Wang J, Wang Y, Wu L, et al. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2020;105(5):e210-e212. doi:10.3324/haematol.2019.222471
- 10.Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. The Lancet Haematology. Published online December 2019:e630-e637. doi:10.1016/s2352-3026(19)30156-5
- Pulmcrit wee: The cutoff razor - April 15, 2024
- PulmCrit Blogitorial – Use of ECGs for management of (sub)massive PE - March 24, 2024
- PulmCrit Wee: Propofol induced eyelid opening apraxia – the struggle is real - March 20, 2024
The treatment was initiated too late after disease onset (20 days)
Hello,
This is such an interesting article, i really liked the aticle.
Time spent reading this is really great.
keep it up!
Source: http://www.mumbaicoworking.com/
Hello,
This is such an interesting article, i really liked the aticle.
Time spent reading this is really great.
keep it up!