 So, the new IDSA guidelines for community acquired pneumonia (CAP) are here. This post will walk us through the guidelines, focusing primarily on issues that relate to critically ill patients. Guidelines can get dull, I've added in GIFs to illustrate my feelings about each recommendation & kept things pretty informal. Come for the GIFs, stay for the medicine.
So, the new IDSA guidelines for community acquired pneumonia (CAP) are here. This post will walk us through the guidelines, focusing primarily on issues that relate to critically ill patients. Guidelines can get dull, I've added in GIFs to illustrate my feelings about each recommendation & kept things pretty informal. Come for the GIFs, stay for the medicine.
Q1: Should gram stain and culture be obtained?
- IDSA recommends this for severe CAP.
- Agree – this is consistent with prior guidelines.
- Not super great evidence behind this, but can occasionally be helpful.
- The IDSA notes this has greatest utility among intubated patients (tracheal aspirate allows for better quality sputum).
- I'd add that if you're obtaining sputum, beware of low-quality sputum samples which can yield spurious results. (Specifically: if the gram stain shows mixed gram-negatives and gram-positives, that doesn't mean you need to add vancomycin.)
Q2: Should blood cultures be obtained at time of diagnosis?
- IDSA recommends this for severe CAP.
- Yup – this is consistent with current practice & guidelines for ICU-level patients.
- This is a “strong recommendation with very low level of evidence” – the yield isn't huge and cultures usually won't change management.
- I would add that if the patient already had blood cultures at another hospital before transfer, you definitely don't need to repeat them!
- To be brutally honest, blood cultures are most important in patients who don't genuinely end up having a pure pneumonia, for example:
- Patients with tricuspid endocarditis and septic emboli (which we are seeing more of due to the opioid epidemic).
- Patients with another source of infection (e.g. intra-abdominal sepsis) who are incorrectly diagnosed with “pneumonia” due to over-reading atelectasis on a chest x-ray.
Q3: Should we test for urinary legionella & pneumococcal antigens?
- Again, IDSA recommends this for severe CAP. This is consistent with prior guidelines.
- IDSA also suggests collecting lower respiratory tract secretions for Legionella culture on selective media or legionella PCR (conditional recommendation, low quality of evidence).
- This is interesting, I've not seen this done.
- I don't think we're going to start PCRing every severe pneumonia patient for legionella. However, if clinical context suggests legionella and the urinary antigen is negative, then PCR could be considered (but realistically, it probably has a long turn-around time).
- Please note that for pneumococcus-positive patients with severe CAP, double-coverage (typically with ceftriaxone plus azithromycin) is still the best therapy. So don't peel off the azithromycin if the urine pneumococcal antigen is positive.
Q4: Should we test for influenza?
- Yep.
- PCR is better than antigen test. Nothing new here.
- Oddly, the IDSA doesn't mention the use of deep respiratory secretion testing for influenza (despite just mentioning this for Legionella!). It is well recognized that a subset of patients with severe influenza may have negative nasopharyngeal swab PCR testing, but subsequently have positive testing using deep respiratory secretions. So, in addition to nasopharyngeal PCR, consider lower respiratory tract sampling in an intubated patient with persistent suspicion for influenza.
Q5: “should serum procalcitonin plus clinical judgement versus clinical judgement alone be used to withhold initiation of antibiotic therapy”
- The wording here is important. Should procalcitonin be used to withhold initiation of antibiotics?
- The obvious answer is no. Procalcitonin is supported by evidence as an antibiotic-stopping tool – not a tool to guide whether antibiotics should be started.
- I've said before, and will say again – procalcitonin has no role in the emergency department management of infection, nor the decision to initiate antibiotics. I continue to believe that it may be useful as a downstream tool (e.g., during ICU days #2-3) to assist in antibiotic discontinuation.
- This frankly seems like a bit of a dodge – the IDSA has asked itself an easy question (which is not the most interesting or important question).
(Q6: this has nothing to do with ICU)
Q7: Should a clinical prediction rule for prognosis plus clinical judgement versus clinical judgement alone be used to determine triage to ward vs higher intensity unit (e.g. ICU or step-down)?
- For patients who don't obviously need ICU, they recommend using the IDSA/ATS minor severity criteria plus clinical judgement.
- Nothing new versus prior guidelines.
- 100% agree, these criteria are evidence-based for risk stratification (more on this in the IBCC here).
- Super important & key point here: that CURB65 and PORT scores are not preferred tools to determine disposition to ICU versus ward (they have been shown to be inferior to the IDSA/ATS minor criteria).
- Note, however, that three IDSA minor criteria (the cutoff they are recommending) has 56% sensitivity and 91% specificity for predicting ICU admission. So these criteria are good, but may miss some patients. When in doubt, a good approach is to:
- #1: Begin with the IDSA/ATS criteria – this is a good starting point to provide a structured and evidence-based approach.
- #2: The final decision is based on taking the IDSA/ATS criteria into the context of overall clinical judgement. If you really think the patient needs ICU, then just send them to ICU (regardless of what the criteria say).
- IDSA/ATS minor criteria are better than SMART-COP.
(Q8, 9.1: nothing to do with ICU)
Q9.2: Recommended antibiotics for severe CAP without risk factors for MRSA or pseudomonas?
- IDSA recommends either a beta-lactam plus macrolide (e.g. azithromycin) or beta-lactam plus fluoroquinolone.
- Beta-lactam is defined here as potentially including: ampicillin-sulbactam, cefotaxime, ceftriaxone, or ceftaroline 600 mg q12.
- They snuck in ceftaroline here! Ceftaroline is a fifth-generation cephalosporin with MRSA activity. Its inclusion here is weird, because they subsequently recommend using either vancomycin or linezolid for patients with MRSA risk factors (not ceftaroline). So, what exactly is the role of ceftaroline… if you're not using it to cover for MRSA??
- The use of beta-lactam plus macrolide for severe CAP is pretty standard, nothing new here.
- Continued recommendation to use beta-lactam plus a fluoroquinolone is disappointing here — I think this is a mistake.
- Fluoroquinolones have lots of toxicity (emerging black-box warnings for neuropathy), and they cause lots of CDiff (more on this here). Fluoroquinolones have largely been eliminated from the University of Vermont ICU and this has correlated with reduced rates of drug-resistant organisms (sorry can't show the data, it's not mine & it's pre-publication).
- A third-generation cephalosporin and fluoroquinolones are two of the highest-risk drugs with regard to CDiff (more on this here). Combining these two drugs together in an ICU is a formula for tons of CDiff.
- The vast majority of evidence supporting double-coverage for CAP is a beta-lactam plus azithromycin. But this data doesn't generalize to beta-lactam plus fluoroquinolone! The authors of the guideline even cite a study showing higher mortality among patients treated with beta-lactam/fluoroquinolone combo versus beta-lactam/macrolide (Vardakas 2017).
- For patients who truly can't tolerate azithromycin and need atypical coverage, I think a better combination is beta-lactam plus doxycycline. Doxycycline has a vastly superior side-effect profile compared to fluoroquinolones. However, overall the vast majority of patients can do fine with azithromycin (which, incidentally, does not cause torsades).
Q10: In the inpatient setting, should patients with suspected aspiration pneumonia receive additional anaerobic coverage beyond standard empiric treatment for CAP?
- IDSA recommends not routinely adding anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected.
- Yay!! Great to see this in print!
- 100% agree, “aspiration pneumonia” is hugely overblown and probably not really a thing (or, perhaps more accurately, all pneumonias are “aspiration pneumonias.”).
- Short story: there's lots of oxygen in the lungs, anaerobic bacteria don't like oxygen, anaerobic bacteria don't cause pneumonia unless there is some sort of anatomic aberration which allows creation of an anaerobic environment (abscess or empyema).
Highlight of the new #IDSA #ATS community-acquired pneumonia guidelines.@PulmCrit has been promoting this for years. ✌️ pic.twitter.com/YI3uaQZdFi
— Fernand Bteich (@fernandbteich) October 10, 2019
Q11: In the inpatient setting, should adults with CAP and risk factors for MRSA or pseudomonas be treated with extended-spectrum antibiotic therapy instead of standard CAP regimens?
- Healthcare Associated Pneumonia (HCAP) is officially dead (byeeee!). This comes as little surprise for a few reasons:
- (a) The ventilator-associated pneumonia guidelines already foreshadowed that HCAP was doomed.
- (b) The whole HCAP category was arbitrary constructed with very poor evidentiary support. Over the years it's been increasingly clear that HCAP was a terrible idea, so it was finally scrapped.
- “We recommend clinicians only cover empirically for MRSA or pseudomonas in adults with CAP if locally validated risk factors for either pathogen are present”
- Yikes! This topic is too hot to handle, so IDSA is punting it to “locally validated risk factors.”
- Um… many hospitals don't have the volume or resources to develop this. Furthermore, generating local data regarding rates of MRSA and pseudomonas will take time and may not be immediately available for most folks.
- “If clinicians are currently covering empirically for MRSA or pseudomonas in adults with CAP on the basis of published risk factors but do not have local etiological data, we recommend continuing empiric coverage while obtaining culture data to establish if these pathogens are present to justify continued treatment for these pathogens after the first few days of empiric coverage (strong recommendation, low quality of evidence)”
- This is more realistic.
- So you can start empiric coverage if risk factors are present, test for the pathogen, and then narrow coverage if drug-resistant organisms aren't found.
- They emphasize the following risk factors: prior culture with MRSA/pseudomonas, recent hospitalization, and exposure to parenteral antibiotics. However, there are certainly many other risk factors.
- They suggest de-escalation in 48 hours if no evidence is found to support the presence of MRSA or pseudomonas.
- This makes lots of sense for MRSA (which can be tested in nasal swab along with other available sources).
- What about de-escalation for pseudomonas in patients who are unable to produce sputum? How are we supposed to exclude pseudomonas in these patients? Asking for a friend.
- MRSA nares swab gets some love here.
- “Treatment for MRSA pneumonia can generally be withheld when the nasal swab is negative especially in non-severe CAP”
- For ICU patients, a negative MRSA nares PCR doesn't necessarily exclude MRSA pneumonia – so judgement is required.
- They qualify this by noting that the positive predictive value of the nares PCR isn't high – so if the swab is positive this doesn't necessarily prove the patient has MRSA pneumonia. Further testing is needed (ideally a lower respiratory tract culture, if obtainable).
Q12: In the inpatient setting, should adults with CAP be treated with corticosteroids?
- “We suggest not routinely using corticosteroids in adults with severe CAP (conditional recommendation, moderate quality of evidence).”
- This is surprising, given several RCTs showing accelerated improvement in severe CAP treated with steroid. Furthermore, the SCCM/ESICM guidelines from 2018 recommend steroid for severe CAP – so this puts the IDSA/ATS guidelines in disagreement with them (29090327).
- They note that some, but not all, meta-analyses have shown that steroids improve mortality. Furthermore, they note that no reported study has shown excess mortality due to steroid.
- With regard to the risk of steroids, they note hyperglycemia (yep) and also possible higher secondary infection rates. I would disagree with this:
- The concept that short-course moderate-dose steroid causes an increase in infection has been debunked in meta-analysis (see myth #2 here). More recently the ADRENAL trial provided additional evidence that moderate-dose steroid doesn't cause infection (while potentially reducing ventilator time and ICU length of stay).
- The evidence they cite to support the concept that steroid increases infection is incredibly shady. They cite a retrospective cohort study and the HYPRESS trial (which I used as a case study for underpowered trials). In HYPRESS, there was a trend towards increased infection in patients treated with steroids which didn't come anywhere close to statistical significance (p=0.26… are you kidding me?)
- They note that steroid use in influenza correlates with worse outcomes. This is true, but whether it's causal or not is unknown. But for now, avoiding steroid in patients with influenza would make sense (more on this in the influenza chapter here).
- So, bottom line, I'm going to side with the SCCM/ESICM guidelines here over the IDSA/ATS guidelines, reproduced here:

Q13: In adults with CAP who test positive for influenza, should treatment regimen include antiviral therapy?
- Yep. For severe ICU-level patients, the answer here is yep.
- (Whether oseltamivir is useful for less sick patients is a more complex question.)
- A more interesting question is whether clarithromycin and naproxen might add anything to oseltamivir, but this wasn't addressed.
Q14: In adults with CAP who test positive for influenza, should the treatment regimen include antibacterial therapy?
- “We recommend that standard antibacterial treatment be initially prescribed for adults with clinical and radiographic evidence of CAP who test positive for influenza in the inpatient and outpatient settings (strong recommendation, low quality of evidence).
- Yep, I agree, patients may often have co-infection with both influenza and bacterial pathogens.
- The guidelines mention that MRSA is associated with influenza, but don't recommend necessarily treating for MRSA (they refer to prior recommendations regarding MRSA made above). Given the known association between influenza and MRSA pneumonia, I would strongly consider empiric MRSA coverage in a critically ill patient with influenza and suspected bacterial pneumonia (but ideally discontinue this in <48 hours as discussed above).
- This is one of the most confusing passages in the guideline, because it kinda seems like they want to cover MRSA but they don't actually make that recommendation:
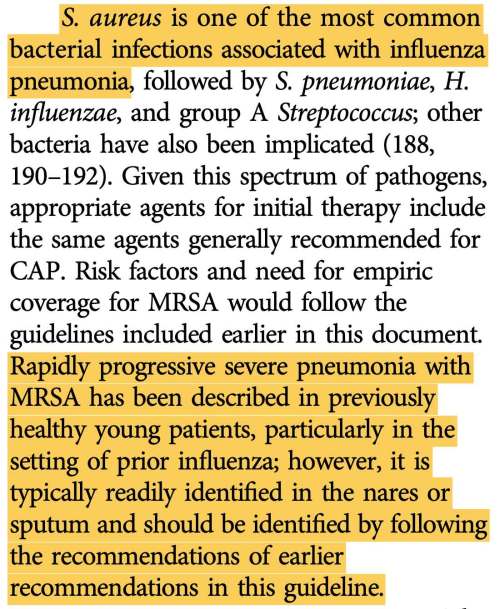
- “However in patients with CAP, a positive influenza test, no evidence of a bacterial pathogen (including a low procalcitonin level), and early clinical stability, consideration could be given to earlier discontinuation of antibiotic treatment at 48 to 72 hours”
- So, if you're worried about bacterial superinfection, start antibiotics up-front and de-escalate as able.
- Love the way they snuck procalcitonin in here as an antibiotic-stopping tool (completely agree with this, but it would be nicer to see procalcitonin brought in through the front door).
Q15: In outpatient and inpatient adults with CAP who are improving, what is the appropriate duration of antibiotic therapy?
- Recommendation: “We recommend that the duration of antibiotic therapy should be guided by a validated measure of clinical stability (resolution of vital sign abnormalities [heart rate, respiratory rate, blood pressure, oxygen saturation, and temperature], ability to eat, and normal mentation), and antibiotic therapy should be continued until the patient achieves stability and for no less than a total of 5 days” (strong recommendation, moderate quality of evidence).
- This recommendation may make sense for outpatients or patients on the ward, but it doesn't work well in the sickest ICU patients.
- Imagine a patient who is intubated due to ARDS as a result of pneumonia. The patient could remain on the ventilator for weeks. This guideline would seem to imply that we should continue antibiotics until the patient is entirely recovered (which doesn't make sense).
- In the discussion, they seem to indirectly imply that a 5-day course is OK for most patients (with 7 days for MRSA or pseudomonas). But this is never rendered as a formal recommendation.
going further
- CAP (IBCC chapter)
- Influenza (IBCC chapter)
- Why fluoroquinolones in the ICU are evil (PulmCrit)
- Evidence-based treatment for severe CAP (PulmCrit)
- Which CAP patients need MRSA coverage? (PulmCrit)
- Pulmcrit wee: The cutoff razor - April 15, 2024
- PulmCrit Blogitorial – Use of ECGs for management of (sub)massive PE - March 24, 2024
- PulmCrit Wee: Propofol induced eyelid opening apraxia – the struggle is real - March 20, 2024
Josh
You wrote a fantastic post. You always do. The cartoons, however, needlessly lengthen the post and are distracting as hell. If someone is past the first quarter of the text, they are not reading to continuously giggle. Please take in good sprit.
Brad.
Good info and memes lighten up the post. Great work as usual
You seem like the kind of person who yelled at Looney Tunes for not employing realistic physics
@Paul
Yup. Mom had issues.
Hospital medicine and pop health. She is ok with that, though.
(were you thinking actuary and i accidentally stumbled on a treatment of CAP site. LOL)
we might have reached a new record for the weirdest argument on the blog?
Great post as always; I mostly agree fully except for the use of steroids. In my opinion a word of caution is still needed. I use them in patients with CAP with a high inflammatory response (usually younger)
In your opinion it would be correct to say that levofloxacin should basically be used only in legionella pneumonia? And what do you think about the use, outside of the ICU, of cycles of CPAP (with continuous flow devices) of 2-3 hours 3-4 times a day alternated with conventional oxygen therapy (in the absence of HFNC) even for patients who maintain adequate saturation in conventional oxygen therapy? Could this promote the recovery of pulmonary lung exchanges or do you think that this kind of CPAP could be detrimental since they generate a rather dry air mixture? Thanks a lot for… Read more »
These recommendations don’t mention covering anaerobes for post-obstructive pneumonia… which obviously isn’t a high oxygen environment. Any thoughts?