
Hydroxychloroquine has been a highly controversial potential treatment for COVID-19. To date, available evidence has consisted of in vitro data as well as some heavily flawed studies from France. A new pre-print from China offers the most meaningful investigation of hydroxychloroquine to date.
design
Tang et al. is a multi-center, open-label RCT involving administration of hydroxychloroquine to 150 patients hospitalized with COVID-19. Sixteen government-designated COVID-19 treatment centers were involved. The dose of hydroxychloroquine used was 1,200 mg daily for three days, followed by 800 mg daily for 2-3 weeks. This is a huge dose of hydroxychloroquine (in comparison, the most commonly used regimen in the United States currently seems to be 800 mg on the first day followed by 400 mg daily for four days). Patients with severe renal or hepatic disease were excluded, to avoid potential drug accumulation.
subjects
Patients were fairly well-matched at baseline. Most patients had relatively mild disease (e.g., with a low rate of dyspnea or hypoxemia). Treatment was initiated late, an average of 16-17 days after disease onset:

Laboratory studies were likewise well matched. Again, patients appear to have had mild disease (with fairly high absolute lymphocyte counts and low levels of C-reactive protein).

primary endpoint
The primary endpoint was viral clearance by 28 days. There was no difference in this endpoint, or in viral conversion at a variety of intermediary time-points.

This isn't a patient-centered endpoint, but it is an extremely important endpoint nonetheless. This endpoint most directly addresses the question: does hydroxychloroquine exert anti-viral activity in vivo? The answer seems to be: nope. Even if the drug were administered too late to affect the clinical course of the infection, if it exerted any anti-viral activity then we might expect to see that effect here. If anything, there might be a trend towards delayed viral clearance in patients treated with hydroxychloroquine.
secondary endpoints
One secondary endpoint was the rate of improvement in symptoms over one month (defined as defervescence, improved oxygen saturation, and disappearance of respiratory symptoms). There was no significant difference in this endpoint:

A retrospective, adjusted analysis did find some improvement in symptoms. However, the validity of an adjusted analysis in a secondary endpoint is dubious.
Numerous lab values were tracked among patients (e.g., C-reactive protein, erythrocyte sedimentation rate, interleukin-6, tumor necrosis factor-alpha). Hydroxychloroquine didn't affect most of these parameters. There was a small difference in C-reactive protein levels, of unclear clinical or statistical significance (p=0.045 in the context of multiple comparisons).
adverse events
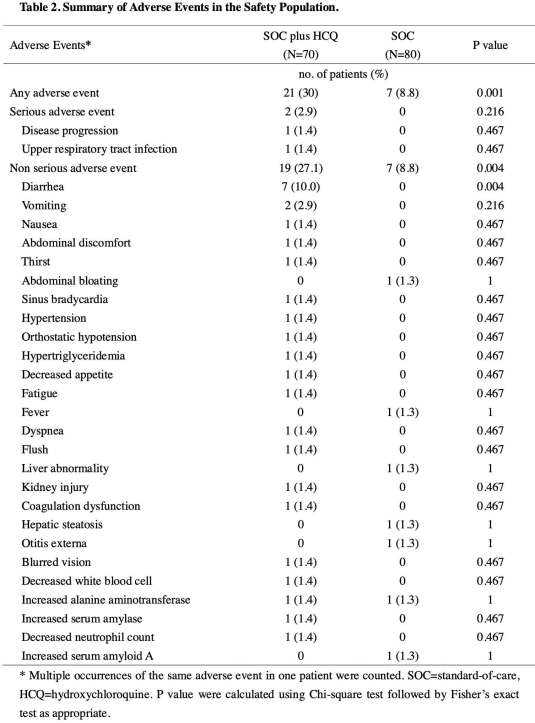
Adverse events were found in 30% of patients treated with hydroxychloroquine, versus 9% of the control group. Although not statistically significant, progression to severe disease occurred at a higher rate in patients receiving hydroxychloroquine.
limitations
The study has major limitations, for example:
- Lack of blinding or placebo control.
- Performance of the study by a contract research organization, rather than directly by the investigators.
- Early termination due to recruitment problems and the impression of benefit (which may have led to under-powering).
- Relatively long delay between symptom onset and treatment initiation (16-17 days).
Nonetheless, this study currently represents the highest available quality of evidence regarding hydroxychloroquine.
timing of antiviral therapies

This is now the third anti-viral therapy to disappoint us within a few weeks (preliminary data on lopinavir/ritonavir and remdesivir were both unimpressive). This raises a question of whether any anti-viral therapies will be beneficial. Especially among the critically ill, patients often present relatively late (at a time-point when viral load is already falling anyway). Much of the pathogenesis of critical illness seems to result from dysregulated inflammation, rather than direct viral cytopathic effect. This raises a question of whether any antiviral treatment will be beneficial for late-presenting patients with severe illness.
Of course, it is possible that earlier use of hydroxychloroquine could be beneficial (e.g., perhaps at the first signs of illness on an out-patient basis). This is under investigation and additional data is likely to be forthcoming soon. Even if this does work in the outpatient clinic, it would probably have little impact on the management of these patients within the intensive care unit.

- This is the first multi-center RCT investigating the use of hydroxychloroquine for COVID-19. 150 adult patients hospitalized with COVID-19 were randomized in an open-label, non-blinded fashion.
- Hydroxychloroquine had no effect on the duration of viral detection (despite the use of high doses and a prolonged 2-3 week course).
- Clinically, there was no discernible beneficial effect from hydroxychloroquine. However, hydroxychloroquine did appear to cause some side-effects (most notably diarrhea).
- Further studies on hydroxychloroquine will likely be emerging soon. For now, the best available evidence does not support the use of hydroxychloroquine in COVID-19. Given evidence of harm without evidence of benefit, it seems prudent to restrict the use of hydroxychloroquine to randomized controlled studies for the time being.
Image credit: Photo by Gabe Pierce on Unsplash
- Pulmcrit wee: The cutoff razor - April 15, 2024
- PulmCrit Blogitorial – Use of ECGs for management of (sub)massive PE - March 24, 2024
- PulmCrit Wee: Propofol induced eyelid opening apraxia – the struggle is real - March 20, 2024
Nice critique Josh, thank you. I am hoping D. Raoult will comment on the Chinese study as well, particularly as your viral load analysis speaks against a theory of increased antiviral effect of the drug if administered early in the disease. Keep up the great work.
Helpful! Thank you. Good analysis of this study . But, so… if it’s not randomized, and there’s not a control group, it’s not a(n) RCT (randomized controlled trial).
Patients were randomized to either the control group which received standard of care without hydroxychloroquine, or an experimental group which received standard of care with hydroxychloroquine. So it was randomized and controlled but not blinded (open-label). The full article describes the randomization process in more detail, I just checked 🙂
Hi Josh, its been a long time but glad to see you doing well. I found myself back in Albany in Nov working at the NYS Dept of health. We are actually working on a few of these studies for covid. Thanks for the post and analysis. Hope you are doing well !
Treatment was initiated late, an average of 16-17 days after disease onset:‘
WTF?!
The limitation of the study will justify its unfavorable result. But, thanks to the author for acknowledging that HCQ is not given at stage 1 of infection which is believed that the drug will play its role at that stage as a zinc ionophore –> leading to less viral load –> and the innate immune system is not overwhelmed (just my idea bcoz the virus will really impair and delay the innate immune system response).
“Treatment was initiated late, an average of 16-17 days after disease onset” – using an anti-viral agent at that stage is just as silly as using Tocilizumab during the viral prodromal phase.
Also- since zinc appears to be the active entity, by inhibiting viral RNA polymerase, and hydroxychloroquine may only be the facilitator of zinc entry, using it without zinc may not be very effective.
This is a case where reading the whole article not just the abstract or summary is important. First, all patients, both HCQ and controls, received additional antivirals. And some of those antivirals such as interferon alpha had already been shown to be effective at treating the disease. So the fact that both groups reached no detectable virus at 28 days may be because of the effectiveness of those other antivirals. The list of those other antivirals appears on page 20. Also, on page 21 the report acknowledges HCQ can have a beneficial effect in reducing the severity of the disease:… Read more »
I fully agree with Mr Clark’s “This is a case where reading the whole article not just the abstract or summary is important.” The article shows that HCQ does not bring so much regarding patients *also treated with antivirals”. On the — very small — subsample which was not treated with other antivirals, the efficacy of HCQ looks like huge: “A significant efficacy of HCQ on alleviating symptoms was observed when the confounding effects of anti-viral agents were removed in the post-hoc analysis (Hazard ratio, 8.83, 95%CI, 1.09 to 71.3).” It looks like “when the confounding effects of anti-viral agents… Read more »
I don´t agree with presentation Title, because journalist can take it literally and say ” HCLQ doesn´t work a clinical study says” … Nobody will give today HCLQ at 15° illnes´s day.Still we need to wait
I’mnot aware of any trials underway with HCQ + zinc unfortunately except in a couple studying it as a prohpylactic. (one of which is only one dose of HCQ every 3 weeks.
Zinc. Without zinc it does not work! Hydroxychloroquine simply opens the cell door to allow zinc access to defeat the virus.
How can such highly educated people doing such an important study no know this?
Absolutely boggle the mind
Because they never had any intention of showing that it works. However it’s already been proven. Raoult has a study with over a 1000 patients. Costa Rica and Turkey have reduced their death rates to nearly nothing, all because they give HCQ , zithromycin and zinc at the onset of the disease.
That’s the proven formula that Dr. Zelenko perfected, and achieved a 95% cure rate in Brazil. Still these “researchers” are paid by the drug companies to show the results they want to show. The VA researchers who claimed that HCQ was harmful worked for Gilead for goodness sake.