
I’ve been yearning to use oral urea for euvolemic hyponatremia for years (e.g. see a post from 2015 here). Urea has been used in Europe for decades, but until very recently it was unavailable in the United States. It has numerous advantages compared to the vaptans (which are extremely expensive and potentially dangerous). Recently, an oral formulation of urea became available in the United States (a commercial formulation marketed under the tradename Ure-Na). Now that we have it, we need to understand how to use this properly.
pharmacology
pharmacodynamics
Oral urea functions as an osmotic diuretic (an “aquaretic”). Ingested urea will be completely excreted by the kidneys. It is excreted along with water, so the ultimate effect is removal of water.

The amount of water removed depends on the urine tonicity. 30 grams of urea is equivalent to 500 mOsm. If the urine osmolarity is 500 mOsm/L, then excretion of the 30 grams of urea will pull a liter of water along with it. Alternatively, if the urine osmolarity is 250 mOsm/L, then 30 grams of urea will require the excretion of two liters of water.
The predicted change in serum sodium can be estimated using the formula below (which approximates total body water as 0.55 multiplied by the patient’s weight in kilograms).

So, for patients with SIADH (who often have a urine osmolarity of ~450 mOsm), the estimated increase in Na following a 30-gram dose of urea is ~3.5 mM (i.e., 3.5 mEq/L). Urea will have a greater impact on patients who have more dilute urine and on patients with lower weights.
One caveat is that the above formula assumes a fixed urine osmolarity. If the cause of hyponatremia is rapidly reversible (say, hypovolemic hyponatremia), then the urine osmolarity may rapidly drop. Dropping urine osmolarity is what the kidney is supposed to do in response to hyponatremia – excrete water. Unfortunately, this could cause over-correction of hyponatremia. Such over-correction may occur with or without urea, but the presence of urea would exacerbate it.
pharmacokinetics
Urea is readily absorbed and freely filtered. In a patient with normal renal function, a dose should be excreted within 12 hours of ingestion.1–3
evidentiary basis
Oral urea for hyponatremia isn’t anything new. It’s been used since the 1970s, and previously recommended in the 2014 European guidelines for management of hyponatremia.4 This section will review some of the more recent evidence.
Croussement et al. 2012: Treatment of the syndrome of inappropriate secretion of antidiuretic hormone with urea in critically ill patients
This is a retrospective description of 24 ICU patients with hyponatremia due to SIADH.5 Two patients were initially admitted due to hyponatremia, whereas most patients developed hyponatremia during their ICU stay (as a complication of acute neurologic or pulmonary disease). Treatment involved water restriction (<1 liter/day) plus oral urea (median dose of 45 grams/day). This achieved a controlled elevation of sodium levels (figure below). Measured urea levels were often quite high, but this didn’t cause any apparent problems.

Rondon-Berrios et al. 2018: Urea for the treatment of hyponatremia

This describes the first year of experience with Ure-Na at four hospitals within the University of Pittsburgh.6 The 58 patients who were included had a broad range of pathologies including SIADH, heart failure, kidney disease, and thiazide-induced hyponatremia. Urea doses ranged between 7.5-90 grams/day. Urea was effective and safe, with no episode of overly rapid correction (defined as >10 mM rise in a day, or >8 mM rise in patients at high risk for osmotic demyelination).
Lockett et al. 2019: Urea treatment in fluid restriction-refractory hyponatremia
This is a retrospective description of 78 episodes of hyponatremia among hospitalized patients which were treated with oral urea.7 SIADH was the most common cause (90%), with others including hypervolemia (9%) and diuretics (8%). The initial dose was 15-90 grams daily (most often 30 grams). No patient developed overcorrection (>10 mM rise in 24 hours). Side effects were minor (6/78 with nausea and 4/78 with hypokalemia). The authors recommended that the starting dose of urea should be at least 30 grams daily.
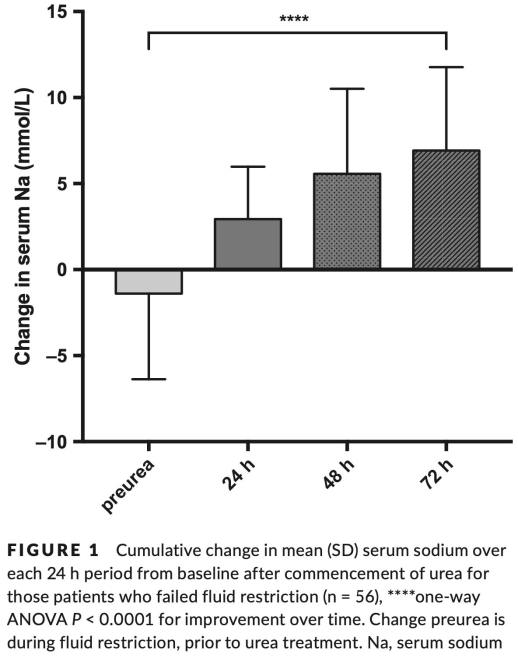
Nervo et al. 2019: Urea in cancer patients with chronic SIAD-induced hyponatremia
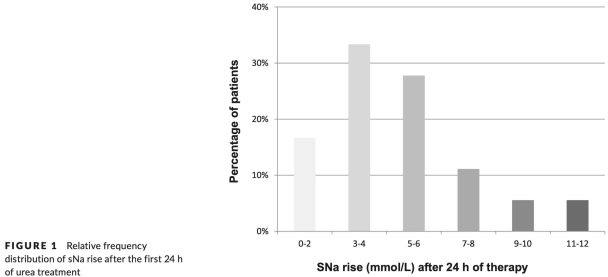
These is a retrospective description of oral urea in 36 patients with SIADH due to malignancy.8 A 30-gram dose was used in 31 patients, and a 15-gram dose in the remaining five. The change in serum sodium over 24 hours is shown below. Some patients did experience an undesirably large increase in sodium, but this was neither common nor extreme.
safety & contraindications
Urea seems to be very safe. Indeed, urea is classified by the FDA as a “medical food” which is “generally recognized to be safe,” so it doesn’t even require a medical prescription.6 The studies above include no examples of severe toxicity from oral urea (side effects seem limited to gastrointestinal effects, including emesis or diarrhea). Administration after meals or in combination with an antacid elixir might improve gastric tolerance, if this is a problem.2
One advantage of urea is that it induces the excretion of a limited and predictable amount of water (in contrast to vaptans, which stimulate an unpredictable amount of water excretion). Since urea causes a limited amount of aquaresis, it is less likely to cause sodium over-correction and osmotic demyelination syndrome. The above studies have substantiated this (although a few patients in Nervo 2019 experienced sodium elevations of 11-12 mM over a day, which is a bit fast).8 One additional safety factor might be that urea itself seems to protect against osmotic demyelination.9 To date, osmotic demyelination syndrome has not been reported with the use of urea.
“Uremic encephalopathy” is a potentially misleading term referring to delirium due to renal failure. The cause of this encephalopathy is not urea itself, but rather a myriad of other toxins which accumulate due to renal failure. Urea functions merely as a bystander which serves to measure renal function, not a causative toxic agent. In fact, the addition of exogenous urea in patients with advanced renal failure has been shown to be safe.10
Urea may be metabolized into ammonia by urease-containing bacteria in the colon, leading to an increase in the patient’s ammonia level. This has led to concern that oral urea could trigger hepatic encephalopathy among patients with cirrhosis. However, analogous to uremic encephalopathy, hepatic encephalopathy is a complex disease state wherein ammonia plays a minor role.11 Thus, oral urea is probably safe for most patients with cirrhosis. Some studies have indeed described the use of urea in small groups of patients with cirrhosis, without inciting encephalopathy.6,12
Urea may be contraindicated in patients with severe renal failure. However, it’s unclear precisely at which GFR or serum urea concentration it might be unwise to use oral urea. The case series from the University of Pittsburgh included patients with baseline blood urea nitrogen levels as high as 62 mg/dL, without any apparent problems.6 Urea therapy often did cause a substantial increase in blood urea nitrogen level, but this was not associated with impaired renal function or with clinical consequences (table below). One potential approach for patients with moderately elevated baseline urea could be to use oral urea and monitor serum urea levels (avoiding trough urea levels >150 mg/dL).13

cost & formulations
Currently, Ure-Na can be purchased in the United States at rates of ~$4 per 15-gram dose (Ure-Na website). Since it's classified as a “medicinal food,” it can be ordered online by anyone. This is about one hundredth of the coast tolvaptan (which costs ~$400 for a single pill).6 For inpatients, this cost is negligible. However, for outpatients on chronic therapy, this will add up (e.g. 45 grams daily for a month = $360).
If Ure-Na isn’t available, other formulations of oral urea might be used (see thread below). The precise dose isn’t critical, so mixing this up on your own could be reasonable. Urea is not classified by the FDA as a drug, which could support the legality of this approach. However, be careful to operate within local regulations (ideally with the collaboration of a pharmacist).
bottom line: nuts & bolts

candidates for oral urea
The use of oral urea is best established among patients with SIADH. However, urea might also be useful in variety of scenarios – especially hypervolemic hyponatremia due to heart failure.1
General criteria for patients who may benefit from oral urea:
- Patients with euvolemic or hypervolemic hyponatremia (especially SIADH or heart failure).
- Absence of severe renal failure or hepatic encephalopathy.
- No immediately reversible cause of hyponatremia (e.g. hyponatremia due to adrenal insufficiency or a medication which can be discontinued). In the presence of a reversible cause, urea could theoretically accelerate the natural rise in sodium, thereby increasing the risk of over-correction.
If the urine concentration is already <<300 mOsm, this may suggest that the kidney has already recovered from whatever process is causing hyponatremia (it has already begun excreting free water on its own). Excretion of dilute urine in a hyponatremic patient generally predicts auto-correction of the hyponatremia (which may occur very rapidly). Urea is probably unnecessary in this scenario and potentially dangerous (it could accelerate auto-correction).
dosing
Doses commonly range between 15-60 grams daily.6,7 A reasonable starting dose may be:
- 15 grams in patients with chronic hyponatremia as a secondary problem (where the goal is to gradually increase the sodium, but there is no urgency in achieving this).
- 30 grams in patients where hyponatremia is a primary problem (so there is some urgency to improving the sodium).2,7
Simultaneous water restriction will help achieve a negative water balance (e.g. <1-1.5 liters/day fluid restriction). If water restriction is impossible, then a higher dose of urea will be necessary to achieve the same net water balance.
monitoring
The optimal intensity of monitoring is unclear. Mild over-correction of serum sodium is uncommon, but possible (e.g. 11-12 mM over 24 hours).8 A dose of oral urea should take effect within 12 hours.1–3 Thus, checking the sodium level after 12 hours might afford an opportunity to titrate therapies, in order to achieve the desired sodium goal at 24 hours after initiation of treatment.
Monitoring of urine output may also be considered. Urea administration should stimulate the production of a moderate, fixed amount of urine. Copious urine production could signal over-correction of sodium due to excessive water excretion (e.g. if urea were mistakenly administered to a patient with a rapidly reversible cause of hyponatremia).
parting thought: is there any role for vaptans anymore?
The vaptans have long been drugs in search of an indication. Their use has largely been supported by relentless promotions by pharma. For example, 2013 guidelines for the management of hyponatremia were largely underwritten by various companies marketing vaptans:14

The use of vaptans is limited by several factors:
- Risk of osmotic demyelination (vaptans may induce uncontrolled water excretion by the kidneys, producing unpredictable increases in sodium level)
- Potential hepatotoxicity
- Enormous cost
The European 2014 guidelines recommended against using vaptans in hyponatremia.4 This has been my practice in the ICU for years, and I haven’t found any situations where vaptans were essential (SIADH may be treated with a combination of furosemide and hypertonic saline or salt tabs – perhaps not as elegant as oral urea, but with sufficient effort it does work).
The traditional argument against urea is that it is unpalatable (and thus incompatible with the delicate “North American palate”).15 Seriously. However, the general consensus on Nephrology twitter seems to be that Ure-Na doesn't taste that bad. Mixology with various sweeteners or juices may further improve the taste. The Europeans have long promoted a concoction known as “Brussels Champagne” which includes 10 grams urea, 2 grams sodium bicarbonate, 1.5 grams citric acid, and 200 mg sucrose.16 However, since this doesn’t originate from the Champagne region of France, it must be referred to as “sparkling urea.”
The introduction of oral urea into our armamentarium may be one final argument against the use of vaptans. As experience accumulates regarding urea, hospitals may wish to consider whether removing vaptans from their formularies could simultaneously reduce costs and improve patient safety.

- Oral urea therapy is finally available in the United States. Urea functions as an osmotic diuretic (a.k.a. aquaretic), with potential use in euvolemic or hypervolemic hyponatremia.
- No RCTs exist comparing oral urea to alternative therapies (e.g. vaptans). However, oral urea is widely regarded as very safe (it’s classified by the FDA as a “medicinal food”). An emerging body of evidence suggests that oral urea is a safe and effective therapy for many forms of hyponatremia.
- Although oral urea is perceived as a “new” therapy in the United States, it has been used for decades in Europe. For example, urea was recommended for management of SIADH in the 2014 European guidelines.4
- Oral urea has important advantages compared to vaptans: it is cheaper and will not cause uncontrolled excretion of free water (thus, urea is unlikely to cause osmotic demyelination syndrome and requires less intensive monitoring than vaptans).
one-minute recap
related
- Hyponatremia chapter in the Internet Book of Critical Care.
- Urea and hyponatremia (Tomoki Tsukahara, Renal Fellow Network)
- Taking control of severe hyponatremia with the DDAVP clamp (PulmCrit). This post includes a discussion of why vaptans are potentially dangerous.
- A better management strategy for symptomatic hyponatremia (EMCrit RACC by Scott Weingart)
- Unconventional therapies for hyponatremia: Thinking outside the collecting duct (PulmCrit). An outside-the-box post on using lactulose for management of hyponatremia. Now that we have oral urea in the United States, lactulose is largely unnecessary for this purpose. However, lactulose will remain useful in patients with hyponatremia and hepatic encephalopathy – who may be poor candidates for oral urea.
(Conflicts of Interest: I never have any conflicts of interest.)
references
- 1.Sterns R, Silver S, Hix J. Urea for hyponatremia? Kidney Int. 2015;87(2):268-270. doi:10.1038/ki.2014.320
- 2.Decaux G, Gankam K, Couturier B, Vandergheynst F, Musch W, Soupart A. Actual Therapeutic Indication of an Old Drug: Urea for Treatment of Severely Symptomatic and Mild Chronic Hyponatremia Related to SIADH. J Clin Med. 2014;3(3):1043-1049. doi:10.3390/jcm3031043
- 3.Annoni F, Fontana V, Brimioulle S, Creteur J, Vincent J, Taccone F. Early Effects of Enteral Urea on Intracranial Pressure in Patients With Acute Brain Injury and Hyponatremia. J Neurosurg Anesthesiol. 2017;29(4):400-405. doi:10.1097/ANA.0000000000000340
- 4.Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170(3):G1-47. doi:10.1530/EJE-13-1020
- 5.Coussement J, Danguy C, Zouaoui-Boudjeltia K, et al. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone with urea in critically ill patients. Am J Nephrol. 2012;35(3):265-270. doi:10.1159/000336716
- 6.Rondon-Berrios H, Tandukar S, Mor M, et al. Urea for the Treatment of Hyponatremia. Clin J Am Soc Nephrol. 2018;13(11):1627-1632. doi:10.2215/CJN.04020318
- 7.Lockett J, Berkman K, Dimeski G, Russell A, Inder W. Urea treatment in fluid restriction-refractory hyponatraemia. Clin Endocrinol (Oxf). 2019;90(4):630-636. doi:10.1111/cen.13930
- 8.Nervo A, D’Angelo V, Rosso D, et al. Urea in cancer patients with chronic SIAD-induced hyponatremia: Old drug, new evidence. Clin Endocrinol (Oxf). 2019;90(6):842-848. doi:10.1111/cen.13966
- 9.Gankam K, Couturier B, Soupart A, Decaux G. Urea minimizes brain complications following rapid correction of chronic hyponatremia compared with vasopressin antagonist or hypertonic saline. Kidney Int. 2015;87(2):323-331. doi:10.1038/ki.2014.273
- 10.Johnson W, Hagge W, Wagoner R, Dinapoli R, Rosevear J. Effects of urea loading in patients with far-advanced renal failure. Mayo Clin Proc. 1972;47(1):21-29. https://www.ncbi.nlm.nih.gov/pubmed/5008253.
- 11.Aldridge D, Tranah E, Shawcross D. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5(Suppl 1):S7-S20. doi:10.1016/j.jceh.2014.06.004
- 12.Decaux G, Mols P, Cauchie P, Flamion B, Delwiche F. Treatment of hyponatremic cirrhosis with ascites resistant to diuretics by urea. Nephron. 1986;44(4):337-343. doi:10.1159/000184016
- 13.Decaux G, Andres C, Gankam K, Soupart A. Treatment of euvolemic hyponatremia in the intensive care unit by urea. Crit Care. 2010;14(5):R184. doi:10.1186/cc9292
- 14.Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-42. doi:10.1016/j.amjmed.2013.07.006
- 15.Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076-1078. doi:10.1681/ASN.2007091042
- 16.Silveira M, Seguro A, da S, et al. Chronic Hyponatremia Due to the Syndrome of Inappropriate Antidiuresis (SIAD) in an Adult Woman with Corpus Callosum Agenesis (CCA). Am J Case Rep. 2018;19:1345-1349. doi:10.12659/AJCR.911810
- Pulmcrit wee: The cutoff razor - April 15, 2024
- PulmCrit Blogitorial – Use of ECGs for management of (sub)massive PE - March 24, 2024
- PulmCrit Wee: Propofol induced eyelid opening apraxia – the struggle is real - March 20, 2024
your electrolyte/renal posts are always my absolute favourites. also love this: “However, since this doesn’t originate from the Champagne region of France, it must be referred to as “sparkling urea.” ”
keep up your monumental works.
thanks Jon-Emile !! the amount of controversy regarding whether americans are able to tolerate PO urea & how to mix it up is pretty hilarious.
Hello. I am not sure your pharmacodynamics calculations, which are valid for effective osmoles, are as valid for an ineffective osmole such as urea. Urea is an important part of the renal concentrating mechanism, as shown by urea transporter knockout mice, but the converse that increasing urea promotes a strong aquaretic is less obvious. Urea is highly permeable at the distal collecting duct, and equilibrates with inner medullary collecting duct [urea]. Thus while the urea transporters are working, it is an ineffective osmole and does not provide aquaresis. We can reconcile this and the clinical findings that high dose urea… Read more »
Great article Dr Farkas, really informative and insightful. I love Urea and use it quite frequently for my SIADH patients. I wanted to let you know of a new, better tasting, lower cost Urea called UreaAide that just hit the market. It is sold by KidneyAide.com. It’s mint flavor and really masks the Urea well and they even have a recipe page!