by Katrina Augustin MD, BSN-RN
Peer Reviewed by Randi Connor-Schuler MD
Drawings by Tammy Lin MD
A Case
A 70 yo woman with a history of hypertension and diabetes presenting via EMS with one week of epigastric pain, fatigue, and dyspnea that worsened acutely today. EMS report worsening dyspnea and hypotension despite non-rebreather and a crystalloid bolus enroute.
Work up in ED notable for CXR showing pulmonary edema, labs with elevated troponin, creatinine and lactate, and ECG with new Q-waves present in inferio-posterior leads.
DISPOSITION DONE: ADMIT TO ICU.
DIAGNOSIS: cardiogenic shock
…Or is there more to her presentation?
Ever have that nagging feeling you are missing something when you admit a patient with unexplained dyspnea and shock that worsens despite all your normal interventions?
Maybe you are.
Think about the Valves
Acute structural heart disease, due to valvular disease or myocardial disruption, is a great masquerader presenting as unexplained dyspnea and hypotension in the setting of acute decompensated heart failure (ADHF) that often responds poorly to standard medical management (PMID: 32314662).
While the diagnosis of structural heart disease can be nuanced and complex, the objective of this post is to focus on simple, efficient identification of acute SEVERE structural heart disease causing decompensation, NOT a comprehensive review of structural heart disease which is beyond the scope of this post.
And, since left-sided, regurgitant valvular disease is not only the most common, but also has the greatest clinical impact, this post will focus specifically on these lesions (PMID: 32314662).
This post will cover:
- Acute Mitral Regurgitation
- Acute Aortic Regurgitation
- Bonus: Acute ventricular septal defects
When assessing a patient with structural heart disease ALWAYS ASK…Is the patient SICK BECAUSE of an acute structural lesion, or SICK WITH an existing structural lesion?
…meaning
Can I explain my patients shock and decompensation on the development of a new structural lesion, OR does my patient have a chronic structural disease with a superimposed systemic illness causing an acute decompensation?
Okay, but why should I care?
Treatment is very different. Acute structural heart disease requires targeted medical stabilization often with the addition of mechanical circulatory support (MCS), early surgical consultation, and definitive surgical treatment while patients with decompensation of their chronic structural heart disease in the setting of a systemic illness such as sepsis, GI bleed, or other pathology need therapy targeted at the underlying systemic process often leading to resolution of their acute presentation (PMID: 32314662).
Part 1: Acute Mitral Regurgitation (MR)
Mechanism & Etiology:
Why is there inadequate coaptation or “coming together” of the valve leaflets?



Pathophysiology (volume overload lesion):
Unlike in chronic MR which allows time for left atrial (LA) dilation to maintain a more normal LA compliance and pressure despite regurgitant volume, in acute MR there is a sudden increase in LA preload due to regurgitant volume during systole leading to increased LA pressure & wall stress, sudden increase in pulmonary capillary wedge (PCWP), and resultant pulmonary edema (PMID: 19564568).
Diagnosis
Suspect acute structural heart disease with pulmonary edema and a normal size cardiac silhouette on CXR. Pulmonary edema may be symmetric or asymmetric. An asymmetric regurgitant jet with flow directed into one pulmonary vein can preferentially lead to asymmetrical pulmonary edema easily confused for PNA (PMID: 19564568).
EKG may have evidence of ischemia if acute MR is secondary to myocardial infarction but could also just depict tachycardia and/or nonspecific T-wave abnormalities.

Formal diagnosis is typically made by comprehensive (quantitative) echocardiography with guidelines for valvular assessment by ASE and EAE which include complex spectral and color wave doppler image acquisition to get precise measurements, perform complex calculations, and integrate multiple diagnostic criteria (PMID: 32314662)–exams that require extensive expertise and time.
Unfortunately, formal echocardiographic assessment is rarely available in the emergency department when a patient is crashing… so how do we make this diagnosis?
…Qualitative point of care ultrasound (POCUS), using skills familiar to emergency medicine physicians.
Comprehensive assessment of native regurgitation adds little to the acute management of severe valvular disorders that are readily identifiable by qualitative assessment (PMID: 19564568). From the front line our focus is acute, severe valvular disease causing hemodynamic decompensation, which frequently can be assessed with practical, simple POCUS techniques using 2D, color flow doppler (CFD), and continuous-wave doppler (CWD) at bedside in the ED.
VALVULAR POCUS MADE SIMPLE
The BASICS
- In 2D assessment: Obtain standard point of care cardiac views (parasternal long, parasternal short, apical 4-chamber, and subxiphoid) to look for structural abnormalities and a visual estimation of cardiac function
- Color Flow Doppler (CFD):
…But before diving into color flow doppler–Let’s talk CFD logistics:



NOW THAT WE DISCUSSED THE BASICS…
ASK: Is this Severe Regurgitation causing my patient to decompensate? To know, look for the following findings:
In 2D:
- Look for evidence of flail leaflet, ruptured papillary muscle, severe valvular retraction, large valvular perforation or significant tethering of leaflet
- Evidence of severe regurgitation plus normal LV/LA size as there is no time for compensatory remodeling

In CFD:
- Vena contracta width 0.7 cm or greater
- Jet area: large central jet that covers >40% of LA area or eccentric jet that reaches posterior wall of LA

Management
General Concepts:
Like every ED patient, first treat C-A-B, then CONSULT…
Circulation
The majority of patients with acute regurgitant lesions will fall into two categories
Category 1: “Warm and wet”
aka Forrester Class II- 9% mortality (PMID: 32280413) with adequate cardiac output and perfusion but evidence of volume overload. Treatment consists of reducing afterload, reducing preload (diuresis/venodilation), and heart rate control.
Afterload Reduction:
Regurgitant lesions are afterload sensitive with worsening regurgitant flow in the setting of high afterload (elevated SVR/aortic impedance). Decreasing afterload will not only decrease regurgitant flow, but also increase SV and CO leading to decreased left ventricular end diastolic pressure (LVEDP), LV wall stress, PCWP, and pulmonary edema. While medications such as nitroprusside and high dose nitroglycerine have been traditionally used in ADHF to reduce afterload, data now also supports the use of dihydropyridine calcium channel blockers (CCB), such as nicardipine or clevidipine, in the treatment of hypertension associated with acute heart failure and these may be easier to use as front line in the emergency department. (PMID 20412469, PMID 24655702, PMID: 2808990, PMID: 33358327, PMID: 8509548).

Preload reduction:
In patients with pulmonary congestion preload reduction is helpful to not only maximize gas exchange but also to optimize myocardial stretch with subsequent improvements in SV and CO. Nitroglycerine, primarily a venodilator at low doses, decreases preload by increasing venous capacitance with increased pooling of blood in the venous system. Starting dose is 5-10 mcg/min and can be titrated q3-5 min. Maximum doses are variable with higher doses also causing arterial vasodilation. In addition, NIPPV (see below) and diuresis using IV loop diuretics can also assist with preload reduction.
Heart Rate Control:
Ideal HR is normal to slightly elevated as slower heart rates can increase time for regurgitant flow. PMID: 32314662
Category 2: “Cold and Wet”
aka Forrester Class IV-51% mortality (PMID: 32280413) with evidence of low cardiac output and pulmonary edema. Treatment of these patients is more complex and focuses on maintaining perfusion with blood pressure and inotropic support, preload reduction as tolerated, and heart rate control.
Defend the MAP

Hypotension in these patients is especially deleterious as they also have elevated left sided filling pressures which in combination with low aortic pressures can lead to severely impaired CPP and worsening myocardial ischemia. Avoid pure vasoconstrictors such as phenylephrine and vasopressin in favor of agents that can also provide inotropy and chronotropy (if needed) such as epinephrine or potentially norepinephrine.
Inotropic support:

PRACTICAL TIPS: No inotrope proven to DECREASE mortality or be superior
…however
Dobutamine, classically thought of as an inodilator with a feared risk of hypotension, was noted in recent review to more frequently cause increased blood pressure then hypotension potentially making it a great option for these patients. Tachycardia and dysrhythmias can more commonly be limiting side adverse effect and it should be used with caution in patients with atrial fibrillation (PMID 29262042).
Milrinone with its delayed onset of action and increased risk of hypotension especially in renal dysfunction may have less utility in the emergency department.
[Editor's Note: I prefer low-dose epi, though I agree with Katrina's comments-SDW]
Preload reduction:
Preload reduction can be performed with NIPPV (see below), as well as potential careful diuresis after supporting MAP and initiating inotropic support in patients in cardiogenic shock.
HR control:
Ideal HR is normal to slightly elevated as slower HRs can increase time for regurgitant flow. However, patients may have poor tolerance of arrythmias such as atrial fibrillation with rapid ventricular rate. Low threshold to consider amiodarone, cardioversion, or even IV digoxin (positive inotrope with rate controlling properties but somewhat limited by delayed onset of action over hours) (PMID: 32314662).
Airway
Does patient have altered mental status from poor perfusion? Are they protecting their airway? Majority will have an intact airway despite dyspnea and shock unless in extremis.
Breathing
Cardiogenic shock is frequently complicated by respiratory failure secondary to increased LVEDP causing pulmonary edema with impaired oxygenation and ventilation.
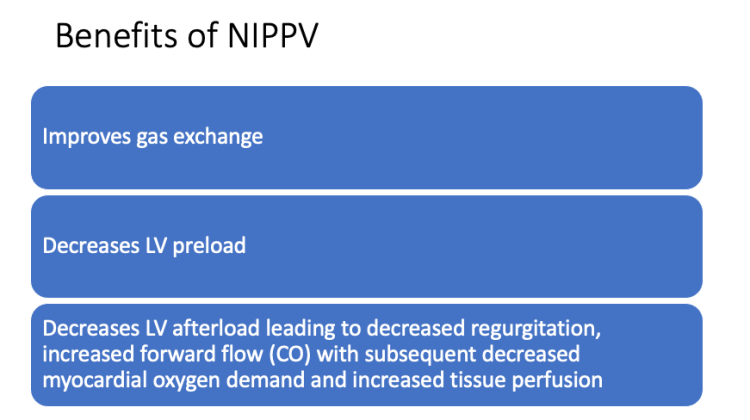
Consider BiPAP with special attention to ePAP to maximize hemodynamic benefits. Can start at iPAP 10 cmH2O / ePAP 5 cmH2O and rapidly titrate up to iPAP 18 cmH2O / ePAP 14 cmH2O as tolerated.
[Editor's note: I personally feel insp. pressure brings nothing to the table in these patients–if they have ventilatory issues, they should be tubed. If it is purely Type I failure, use CPAP, not BIPAP]
Caution with intubation as these patients are high risk for cardiac arrest and hemodynamic decompensation with intubation. If intubation is needed, resuscitate first and consider preoxygenation with NIPPV and use of more cardiac stable induction medications.
[Editor's note: please consider hemodynamic neutral intubations in these patients]
Then CONSULT:
Many patients with acute structural heart disease fail to stabilize with medical therapy alone and will require mechanical circulatory support (MCS) to help stabilize them as a bridge to definitive surgical or interventional treatment. Early consultation with cardiothoracic surgery and potentially interventional cardiology (based on your institution and the patient’s needs) is imperative. Stabilization with medical therapy plus MCS can turn a high risk emergent MV surgery into a lower risk semi-elective operation. (PMID: 32314662)
…And if you really want to speak your consultant’s language and make your MDM sound way smarter just mention their SCAI stage of cardiogenic shock.

Classification of cardiogenic shock with the SCAI Classification (Society for Cardiovascular Angiography and Interventions) was created to optimize patient care through a uniform system to identify patients at risk from dying from cardiogenic shock. SCAI classifications range from A (at risk for shock) to E (extremis).
Common MCS devices used for stabilization of severe MR
- IABP: uniquely able to reduce LV afterload and increase CPP while not decreasing MAP
- Impella: able to augment forward flow, decrease LVEDP, and pulmonary congestion
- Peripheral veno-arterial ECMO can be considered but increases LV afterload and can worsen LVEDP, and pulmonary congestion
Part 2: Acute Aortic Regurgitation (AR)
Mechanism & Etiology:

Pathophysiology of Acute AR (volume overload lesion):
Unlike in chronic AR where there is time for compensatory LV dilation to maintain stroke volume and cardiac output while maintaining a more normal LVEDP, in acute AR the sudden increase in LV volume from the regurgitant flow into the noncompliant LV leads to sudden increase in LVEDP, left atrial pressure/PCWP, and subsequent pulmonary edema leading to the classic presentation of dyspnea, volume overload, and CS (PMID: 32314662).
Diagnosis:

ECHO
As with MR, diagnosis is formally made via formal echocardiography; however, qualitative POCUS can be used in the ED to identify severe acute aortic regurgitation. See Valvular Basics above.
As always ask: Is this Severe Regurgitation causing the patient to decompensate?
In 2D: Look for flail leaflets, coaptation defects, aortic dissection flap
In CFD:
- VC width 0.6 cm or greater
- VC to left ventricular outflow tract (LVOT) diameter ratio of equal to or > .65
In Continuous wave doppler (CWD) (or pulse wave doppler (PWD):
- Measure pressure half time (PHT), if <200 ms then indicative of severe AR
…CWD? PHT? WHAT….? Back to the BASICS.
First, place color flow doppler over the LVOT in apical 5 chamber view then identify the regurgitant jet back into the LV (red jet), then place continuous wave doppler over the regurgitant jet and measure envelope from peak to end of envelope: less than 200 ms indicative of severe AR.

Management: (see general concepts above)
As in acute MR, definitive treatment is often surgical and the goal of medical management is stabilization.
So immediate goals are…
- Minimize afterload
- Reduce congestion
- Maintain lowest MAP that is still able to provide adequate end organ perfusion
Much of the treatment is similar to acute MR (noted above) so only key differences are highlighted below.
Aortic dissection:
AR due to aortic dissection should be treated with standard afterload reduction to SBP of 100-120 mm HG as usual; however, anti-impulse propagation therapy with B-Blocker to usual goal of HR <60 can block compensatory tachycardia, increase time in diastole, and increase regurgitant volume leading to marked hypotension so routine B-Blockers not recommended in these patients (PMID: 24603191). Definitive treatment is surgical repair.
Endocarditis
causing acute severe AR should receive standard therapies including blood cultures and antibiotics in addition to management of severe AR.
MCS devices are CONTRAINDICATED in acute aortic regurgitation
[Editor's note: well… not so much once they are dead. See Shinar and Bellezzo paper on topic–Aortic Dissection case was Sharp ECPR's 1st save)
- IABP/ECMO contraindicated in AR as can precipitate worsening regurgitation in the setting of an incompetent AV with subsequent increased LVEDP and pulmonary edema.
- Impella is relatively contraindicated as it can theoretically lead to recirculation in setting of incompetent AV.
Early consultation with cardiothoracic surgery and potentially interventional cardiology (based on your institution and the patient’s needs) is imperative.
Part 3: Acute Ventricular Septal Defect (VSD)
…you are probably wondering why VSDs are included in this post, after all this is no valvular emergency! True, but it is a form of structural heart disease that shares much of the same presentation, urgency, and treatments of the above regurgitant lesions and is also associated with “regurgitant flow” in a way otherwise known as SHUNTING. Patients classically present with sudden onset dyspnea, pulmonary edema, and hemodynamic collapse like the above regurgitant lesions.
Etiology:
Unlike chronic VSDs, which are mostly congenital, acute VSDs are primarily caused by mechanical complications associated with a late presentation of acute myocardial infarction.
Risk Factors:
- Older age
- Delayed presentation after myocardial infarction
- Female gender
- Delayed reperfusion, no reperfusion, and potentially thrombolytic therapy
- No previous myocardial infarction (think likely less collateral circulation)
Culprit Vessels:
- RCA infarction presenting as inferior MI potentially with concomitant RV infarction with an inferior septum VSD
- LAD infarct causing an anterior MI with an apical VSD
Pathophysiology:
In an acute VSD, the LV (higher-pressure system) will be offloaded into the RV leading to L to R shunting (unlike in chronic VSDs who often develop pulmonary hypertension and can lead to R to L shunting or bidirectional shunting). L to R shunting leads to RV pressure and volume overload which can cause dilation and dysfunction of the RV. As with the above lesions, LV ejection fraction will over-estimate systolic function in the setting of significant shunting (PMID: 32314662).
Diagnosis:
EKG findings may be nonspecific in acute VSD; however, could depict findings consistent with late presentation of myocardial infarction including q-waves.
Echo
Formal diagnosis is made via formal echocardiography; however, like with valvular disease, bedside POCUS can be used to identify acute VSDs causing hemodynamic instability.
CFD is the most valuable tool for diagnosis of acute VSDs with a reported sensitivity of 95%. It can depict size and location of defect as well as provide hemodynamic information regarding VSD (PMID 29261884).
In 2D:
Assess for evidence of septal defect
Video 1: Clip depicts VSD in 2D ultrasound. Video clip courtesy of Christopher Clark MD, Henry Ford Hospital
Perform bubble study
… WAIT…. do WHAT in the ED?
Think of it as checking your central line placement with POCUS. EASY RIGHT?
To perform a bubble study, inject saline agitated with air creating echogenic micro-bubbles (contrast) and assess to see if they cross from the RV to LV. With an intracardiac shunt, bubbles classically appear on the left side within 3-4 heartbeats. Due to higher LV to RV pressure gradient in acute VSD may see “negative RV contrast” or washout of the echogenic bubbles in the RV due to blood traversing through the IVS from the LV to the RV (PMID: 6848222).
Sensitivity of the study can be increased by having patient perform a Valsalva maneuver which transiently increases right sided pressures increasing the likelihood of R to L shunting of bubbles (PMID: 25911721).

In CFD:
Place CFD over septum and look for flow through septum, typically L to R flow in setting of acute VSD
Video 2: Clip depicts VSD with CFD illustrating flow through the IVS. Video clip courtesy of Christopher Clark MD, Henry Ford Hospital
Management:
Similar to the above regurgitant lesions, acute VSDs are AFTERLOAD SENSITIVE with increased LV afterload causing worsening LàR shunting.
General Concepts:
- Minimize afterload
- Maintain lowest MAP that is still able to provide adequate end organ perfusion to minimize shunting
Goal of medical therapy is to temporize hemodynamics until definitive therapy can be performed. Depending on hemodynamic profile/Forrester classification patient may require vasodilators, inotropes, preload reduction/diuresis, and even vasoconstrictors (avoid pure vasoconstrictors). See MR management for more details as treatment is similar (PMID: 32314662).
Use NIPPV with care as can be helpful to decrease LV preload and afterload; however, can cause increased RV AFTERLOAD which can be detrimental for a failing RV in the setting of significant RV pressure/volume overload from left to right shunting.
Early Consultation with cardiothoracic surgery and interventional cardiology is imperative as many of these patients will need MCS to help stabilize them till definitive surgical repair is performed.
MCS:
- IABP: recommended as it can decrease LV afterload while maintaining MAP and CPP
- Peripheral V-A ECMO can be used for patients in extremis; however, carries with it the risk of increased LV afterload which can worsen LàR shunting and thus may require additional LV unloading with an impella (ECPella)
- Impella can be effective to unload LV but is contraindicated per FDA as it could theoretically cause a Rà L shunt across the VSD (PMID: 32314662).
Definitive repair is best accomplished via DELAYED surgical patch repair as initially tissue is friable and prone to form a residual VSD after the scar tissue contracts. For patients to unstable to await surgical repair despite medical management and MCS, attempted percutaneous closure of the VSD can be used as either a bridge to definitive therapy or as definitive therapy depending on the lesion (PMID: 32314662).
Take Home Message on regurgitant structural heart disease lesions
- Acute MR, AR, and VSD are highly dynamic processes influenced by loading conditions such as hypertension and volume overload which can worsen regurgitation and shunting
- Regurgitant lesions are afterload sensitive, so it is imperative to target the lowest MAP needed for organ perfusion to minimize regurgitant flow
- Left ventricular ejection fraction will overestimate function with regurgitant lesions
- Early consultation is imperative as most of these patients need MCS in addition to medical therapy to stabilize them till definitive surgical treatment
Additional References/Resources
RECAPEM, Valvular Emergencies Part 1 and Valvular Emergencies Part 2
CARDIOSERV, 9 Steps to Perform an Echo Bubble Study
123 Sonography, Quantification of Mitral Regurgitation
Larry X. Nguyen, Benjamin Kohl, How do I manage acute heart failure? Editor(s): Clifford S. Deutschman, Patrick J. Neligan, Evidence-Based Practice of Critical Care (Third Edition), Elsevier, 2020, Pages 366-370.e1, ISBN 9780323640688
Acute Valvular Emergencies: Pearls and Pitfalls
Additional New Information
Post-Publication Peer Review
Lewis Mclean Writes:
I should note that I'm an Australian Intensivist with subspecialty training in critical care echocardiography and thus am not quite as familiar with USA things as I might be…
Firstly – in acute MVR the LVEF will be increased, i.e. hyperdynamic, with an EF of 70-75% being the classical teaching. I did not feel this was adequately emphasised – a “normal EF” as described in the podcast is a failing LV. I liken it to a “normal” PCO2 in asthma, or DKA – a sign that compensatory mechanisms are failing.
Secondly, the use of bubble study in VSD is usually limited to congenital cases, where the chance of right to left or bidirectional shunt is much higher. AMI related VSD don't fall into this category, and colour Doppler over the IVS is the best discriminator..
I feel somewhat as I'm nitpicking, but I felt I should give some feedback as a quality assurance…
Regards,
Lewis
More on EMCrit
Additional Resources
- CV-EMCrit Wee – MCS Minute: ECMO and the DO2/VO2 ratio with Trina Augustin - January 31, 2024
- CV-EMCrit 327 – Acute Valve Disasters Part 2 – Management of Critical Aortic Stenosis - July 1, 2022
- EMCrit 321 – CV-EMCrit – Acute Valve Disasters – Critical Aortic & Mitral Regurgitation and Bonus: VSDs with Trina Augustin - April 7, 2022







Your blog are completely awesome and share worthy. I really appreciate your efforts that you put on this. Keep sharing.
new holland 3630
Thank you! So glad it was helpful! More to come.
We should be picking these up more often, now that more EPs are performing echo. A couple of cases that I was involved with. The VSR ventricular septal rupture was a stunning pick up. Since then I have had a flail mitral valve and a free wall rupture.
Heartbreak: A case of post‐infarction cardiogenic shock – Mukherjee – 2019 – Australasian Journal of Ultrasound in Medicine – Wiley Online Library
Is it septic or cardiogenic shock? Papillary muscle rupture masquerading as sepsis – Mukherjee – 2019 – Australasian Journal of Ultrasound in Medicine – Wiley Online Library
Great education ever time…. Easily applied to the bedside
I had a question regarding mitral regurg emergencies. Is heart rate as paramount to control (keep slightly fast) in these scenarios as the regurgitation is occurring in systole? Thanks
Interesting question! With bradycardia their is increased time in diastole leading to increased LV end diastolic volume/size, this in turn worsens MR (think increased volume loading/ further distending the mitral valve annulus at the end of diastole and increased regurgitation of this volume during systole).
In acute mitral regurgitation I understand the concept of reducing time in diastole to prevent LV bloating and reducing regurgitant volume, but compared to aortic regurgitation this seems like it could be self limiting for coronary perfusion which occurs primarily in diastole for the LV. In acute MR is there perhaps a balancing point between time to perfuse the left heart and the pressure volume relationship of the LV. In acute AR it seems to be more paramount given the diastolic nature of the regurgitation and a reduced coronary pressure pressure head. Could anyone help wrap my head around this?… Read more »
Sorry I just read the past comments….please disregard
Here in Germany we commonly use Urapidil and Dihydralazine in the ICU. Any idea, why Nitroprusside and Ca-Blockers are preferred in the US?